 |
 |
| Tuberc Respir Dis > Volume 86(2); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Notes
Authors’ Contributions
Conceptualization: Lee SY, Yoon SH. Methodology: Lee SY, Yoon SH, Hong H. Formal analysis: Lee SY, Yoon SH, Hong H. Data curation: Lee SY, Yoon SH. Project administration: Lee SY. Resources: Lee SY. Software: Lee SY, Hong H. Supervision: Lee SY. Validation: Lee SY, Yoon SH, Hong H. Visualization: Lee SY, Yoon SH. Investigation: Lee SY, Yoon SH, Hong H. Writing - original draft preparation: Lee SY, Yoon SH, Hong H. Writing - review and editing: Lee SY, Yoon SH, Hong H. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Figure S1.
Fig. 2.

Fig. 3.
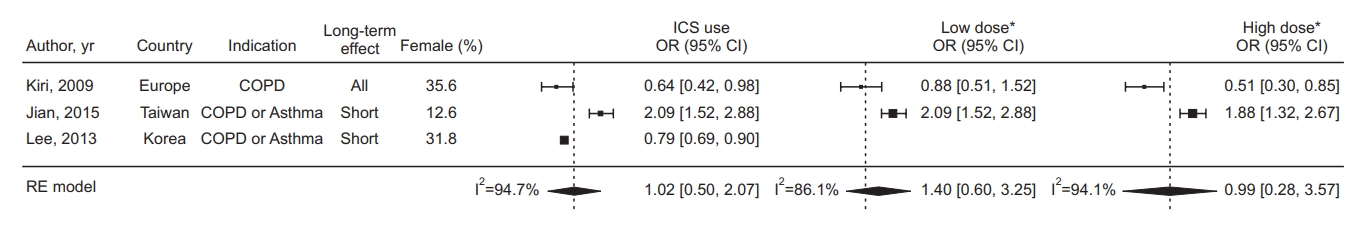
Fig. 4.
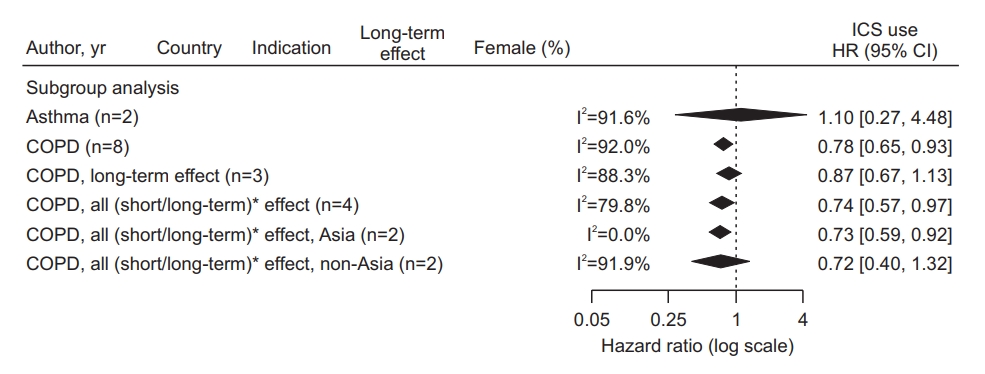
Table 1.
| Study | Country | Study design | Patient collection | Recruitment period | Data source | No. of subjects* | Mean age, yr | Male sex, % | Ever-smoker† |
|---|---|---|---|---|---|---|---|---|---|
| Suissa et al. (2020) [11] | Canada | Cohort study | Retrospective | 2000-2014 | Provincial population-based database | 63,276 | 71 | 53 | Not available |
| Husebo et al. (2019) [14] | Norway | Cohort study | Prospective | 2006-2009 | Multicenter cohort | 712 | 64 | 60 | 100% (51%:49%) |
| Raymakers et al. (2019) [12] | Canada | Cohort study | Retrospective | 1999-2007 | Provincial population-based database | 39,676 | 71 | 47 | Not available |
| Lee et al. (2018) [19] | Korea | Nested case control study‡ | Retrospective | 2004-2013 | Sample cohort of national health insurance | 1,325 (265:1,060) | 64 | 78 | 52% (28%:24%) |
| Sandelin et al. (2018) [13] | Sweden | Cohort study | Retrospective | 1999-2009 | Nationwide population-based database | 19,894 | 68 | 47 | Not available |
| Sorli et al. (2018) [15] | Norway | Cohort study | Prospective | 1995-1997 | Multicenter cohort | 3,041 | 61 | 53 | Not available (mean pack- year, 22) |
| Wang et al. (2018) [16] | Taiwan | Cohort study | Retrospective | 2001-2005 | Claim database of national health insurance§ | 41,438 | 50-59∥ | 47 | 0% |
| Liu et al. (2017) [20] | Taiwan | Cohort study | Retrospective | 1997-2009 | Claim database of national health insurance§ | 13,686 | ≥60∥ | 0 | Not available |
| Jian et al. (2015) [17] | Taiwan | Nested case control study‡ | Retrospective | 2003-2010 | Claim database of national health insurance§ | 3,965 (793:3,172) | 72 | 87 | Not available |
| Kok et al. (2015) [21] | Taiwan | Cohort study | Retrospective | 2001-2008 | Claim database of national health insurance§ | 19,849 | 53 | 46 | No¶ |
| Lee et al. (2013) [22] | Korea | Nested case control study‡ | Retrospective | 2007-2010 | Claim database of national health insurance | 46,225 (9,177:37,048) | 68 | 68 | Not available |
| Kiri et al. (2009) [18] | UK | Nested case control study‡ | Retrospective | 1989-2003 | National general practice research database | 1,597 (127:1,470) | 71 | 64 | 100% (100%:0%) |
| Parimon et al. (2007) [5] | USA | Cohort study | Prospective | 1996-1999 | Multicenter cohort | 10,474 | 64 | 97 | 88% (34%:54%) |
Table 2.
| Variable |
Subgroup analysis |
Meta-regression |
||||
|---|---|---|---|---|---|---|
| No. of studies | HR (95% CI) | I2* | p-value | I2† | ||
| Indication | 0.1704 | 93.8% | ||||
| Asthma | 2 | 1.10 (0.27-4.48) | 91.6% | |||
| Chronic obstructive pulmonary disease | 8 | 0.78 (0.65-0.93) | 92.00% | |||
| Lengths of latency period‡ | 0.3564 | 86.8% | ||||
| All (short+long) | 5 | 0.88 (0.57-1.36) | 92.9% | |||
| Long | 4 | 0.82 (0.63-1.06) | 85.4% | |||
| Unknown | 1 | 0.40 (0.17-0.94) | - | |||
| Region | 0.6054 | 95.1% | ||||
| Asia | 4 | 0.88 (0.49-1.60) | 86.3% | |||
| Non-Asia | 6 | 0.78 (0.62-1.00) | 95.7% | |||
Table 3.
| Study | Disease |
Inclusion criteria |
Exclusion criteria |
|||||
|---|---|---|---|---|---|---|---|---|
| Age, yr | Patient selection | New diagnosis | Previous history of cancer | Former ICS users | Asthma* | Other | ||
| Suissa et al. (2020) [11] | COPD | ≥50 | Prescription-based (long-acting BD ≥3 times a year) | New drug users | Lung cancer | Yes | No | Follow-up <1 year† |
| Husebo et al. (2019) [14] | COPD | 40-76 | Physician-diagnosed or spirometry-based‡ | No | Any cancer | Not mentioned | Yes | Active inflammatory disorders, COPD exacerbation within 4 weeks of entry |
| Raymakers et al. (2019) [12] | COPD | ≥50 | Prescription-based (short-acting BD ≥3 times a year) | New drug users | Lung cancer | Not mentioned | Subgroup analysis | Follow-up <1 year†, lung cancer within a year after entry |
| Lee et al. (2018) [19] | COPD | 30-89 | ICD code & prescription-based (inhaled drugs ≥twice) | New diagnosis & new drug users | Lung cancer | Yes | No | |
| Sandelin et al. (2018) [13] | COPD | No | ICD code-based (≥once) | No | Not mentioned | Not mentioned | No | |
| Sorli et al. (2018) [15] | Chronic airway inflammation | ≥20 | Patient-reported (cough/sputum for 3 months) or spirometry-based§ | No | Lung cancer (before 2002) | Not mentioned | Not applicable | |
| Wang et al. (2018) [16] | Asthma | 40-70 | ICD code-based (≥once [ward] or ≥3 times in 3 months [outpatient]) | New diagnosis | Lung cancer | Yes | Not applicable | Lung cancer within 2 years after entry†, smokers |
| Liu et al. (2017) [20] | COPD | ≥40 | ICD code-based (≥once [ward] or ≥twice [outpatient] a year) | New diagnosis | Lung cancer | Not mentioned | Yes | |
| Jian et al. (2015) [17] | COPD, asthma | ≥20 | ICD code-based (≥once) | New diagnosis | Lung cancer | Not mentioned | Not applicable | Missing data, lung cancer within 2 years after entry† |
| Kok et al. (2015) [21] | Asthma | ≥20 | ICD code-based (≥3 times a year) | New diagnosis | Any cancer | Yes | Not applicable | Missing data, ICD code <3 times a year |
| Lee et al. (2013) [22] | COPD, asthma | 20-120 | Prescription-based (inhaled drugs for ≥30 days) | New drug users | Any cancer | Not mentioned | Not applicable | |
| Kiri et al. (2009) [18] | COPD | ≥50 | Physician-diagnosed (ex-smoker COPD) & prescription-based (inhaled drugs within 6 months of enrollment) | New diagnosis & new drug users | Lung cancer | Not mentioned | Not mentioned | Cystic fibrosis |
| Parimon et al. (2007) [5] | COPD | ≥40 | Physician-diagnosed or patient-reported (chronic lung disease) or prescription-based (BD within 1 year before enrollment) | No | Lung cancer | Not mentioned | Not mentioned | |
Table 4.
| Study | ICS exposure | Proportion of ICS users* | Definition of ICS users | Definition of non-ICS users | Period of ICS use | ICS drugs | Median ICS dose† |
|---|---|---|---|---|---|---|---|
| Suissa et al. (2020) [11] | Time-dependent variable | 63% (40,164/63,276) | Person time under ICS exposure | Person time of non-ICS users & before 1st ICS exposure | During the study period | Beclo., Budeso., Triam, Flutica., Cicleso., Fluniso. | Daily dose, 0-500 μg‡ |
| Husebo et al. (2019) [14] | Fixed variable | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Raymakers et al. (2019) [12] | Time-dependent variable | 71% (28,314/39,676) | Person time under ICS exposure | Person time of non-ICS users & before 1st ICS exposure | During the study period | Not mentioned | Daily dose, 640 μg |
| Lee et al. (2018) [19] | Fixed variable | 63% (833/1,325) | ICS prescription ≥twice | No ICS prescription or ICS prescription once | During the study period | Beclo., Budeso., Triam, Flutica., Cicleso., Fluniso. | Cumulative dose, 90,000 μg |
| Sandelin et al. (2018) [13] | Time-dependent & fixed variables | Not mentioned | Not mentioned | Not mentioned | During 2 years before entry | Not mentioned | Not mentioned |
| Sorli et al. (2018) [15] | Fixed variable | 36% (1,095/3,041) | Patient-alleged ever regular ICS users | No ICS users or ICS irregular users | Lifetime | Beclo., Budeso., Flutica. | Not mentioned |
| Wang et al. (2018) [16] | Fixed variable | 10% (4,210/41,438) | ICS prescription >28 days/month in ≥4 consecutive months | No ICS prescription or ICS prescription <4 consecutive months | During the study period | Beclo., Budeso., Triam, Flutica., Fluniso. | Not mentioned |
| Liu et al. (2017) [20] | Fixed variable | 9% (1,290/13,686) | ICS prescription for >28 days | No ICS prescription | During the study period | Budeso., Flutica. Beclo., Budeso., | Cumulative dose, 39,480 μg |
| Jian et al. (2015) [17] | Fixed variable | 12% (492/3,965) | Not mentioned | Not mentioned | During 2 years before entry | Flutica., Cicleso., | Cumulative dose, 90,000 μg |
| Kok et al. (2015) [21] | Fixed variable | 11% (2,117/19,849) | ICS prescription ≥6 times a year | No ICS prescription | During the study period | Beclo., Budeso., Flutica. | Not specified |
| Lee et al. (2013) [22] | Fixed variable | 30% (14,017/46,225) | ICS prescription for ≥30 days | No ICS prescription or ICS prescription <30 days | During 1 year before entry | Beclo., Budeso., Triam, Flutica., Cicleso., Fluniso. | Cumulative dose, 90,000 μg |
| Kiri et al. (2009) [18] | Fixed variable | 74% (1,176/1,597) | ICS prescription ≥3 times | - | Within 6 months of entry | Not mentioned | Not specified |
| Parimon et al. (2007) [5] | Fixed variable | 5% (517/10,474) | ≥80% adherent | No ICS prescription & <80% adherent | During the 180 days before entry | Beclo., Triam, Flutica., Fluniso. | Daily dose, 300 μg |
Table 5.
| Study | Lung cancer incidence, % | Follow-up duration, yr | Statistics | Summary | Adjusted confounders | Latency between ICS exposure and lung cancer occurrence, yr* | Immortal time bias† |
|---|---|---|---|---|---|---|---|
| Suissa et al. (2020) [11] | 5.9‡ | Mean, 4.7 | Time-dependent Cox regression | aHR, 1.01 (0.94-1.08) | Age, sex, comorbidities | 1 | Adjusted |
| Husebo et al. (2019) [14] | 4.4 | Mean, 9 | Cox regression | aHR, 0.40 (0.17-0.93) | Age, sex, smoking, body composition, emphysema | No | No |
| Raymakers et al. (2019) [12] | 2.5‡ | Mean, 5.2 | Cox regression | aHR, 0.70 (0.61-0.80) | Age, sex, region, income, hospitalization, comorbidities, medication | 1 | Adjusted |
| Lee et al. (2018) [19] | 2.5 | Mean, 4 | Cox regression | aHR, 0.74 (0.57-0.96) | Income, smoking, body mass index, comorbidities | No | No |
| Sandelin et al. (2018) [13] | 3.0 | Not mentioned | Cox regression | aHR, 0.52 (0.37-0.73) | Age, asthma, medication | No | Potentially adjusted |
| Sorli et al. (2018) [15] | 3.4 | Not mentioned | Cox regression | aHR, 0.97 (0.61-1.54) | Age, sex, smoking, forced expiratory volume in one second | No | No |
| Wang et al. (2018) [16] | 1.8 | Not mentioned | Cox regression | aHR, 0.42 (0.31-0.56) | Age, sex, allergic status, and comorbidities | 2 | No |
| Liu et al. (2017) [20] | 2.2 | Median, 9.8 | Cox regression | aHR, 0.45 (0.21-0.96)§ | Age, income, comorbidities | No | No |
| Jian et al. (2015) [17] | 20.0 | Mean, 3.9 | Conditional logistic regression | aOR, 2.09 (1.52-2.88) for low ICS & 1.88 (1.32-2.66) for high ICS | Region, income, health care utility, comorbidities, aspirin use | 2 | No |
| Kok et al. (2015) [21] | 6.0 | Mean, 3.5 | Cox regression | aHR, 2.23 (1.31-3.79) | Age, sex, comorbidities, smoking-related diagnoses, asthma medication | No | Adjusted |
| Lee et al. (2013) [22] | 24.8 | Not mentioned | Conditional logistic regression | aOR, 0.79 (0.69-0.90) | Bronchodilator and oral steroid use | No | No |
| Kiri et al. (2009) [18] | 8.0 | Not mentioned | Conditional logistic regression | aOR∥, 0.64 (0.42-0.98) for ICS & 0.50 (0.27-0.90) for LABA/ICS | Duration of both smoking cessation and COPD, comorbidities, medication | No | No |
| Parimon et al. (2007) [5] | 4.0 (2.4‡) | Median 3.8 | Cox regression | aHR, 0.39 (0.16-0.96)¶ & 0.41 (0.13-1.31)¶ | Age, smoking, history of malignancy other than skin cancer, comorbidities, bronchodilator use | 1 (subgroup analysis) | No |
* The latency indicated the minimum interval that ICS can affect lung cancer development. The authors assumed that ICS might not affect a biological plausibility of lung cancer within 1 year before the establishment of lung cancer.
† The immortal time bias occurs when unexposed period to ICS (before the first ICS exposure) is assigned exposed period. The bias can overestimate time considered as exposed.
References
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Supplement1
Supplement1 Print
Print Download Citation
Download Citation




