|
 |
| Tuberc Respir Dis > Volume 87(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Authors’ Contributions
Conceptualization: Yeo HJ, Cho WH. Methodology: Kim K. Formal analysis: Kim K. Data curation: Jang JH, Park S, Lee SH, Park O, Kim TH. Validation: Yeo HJ, Cho WH. Writing - original draft preparation: Kim J, Yeo HJ. Writing - review and editing: Cho WH. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Table S1.
Supplementary Table S3.
Supplementary Table S4.
Fig. 1.
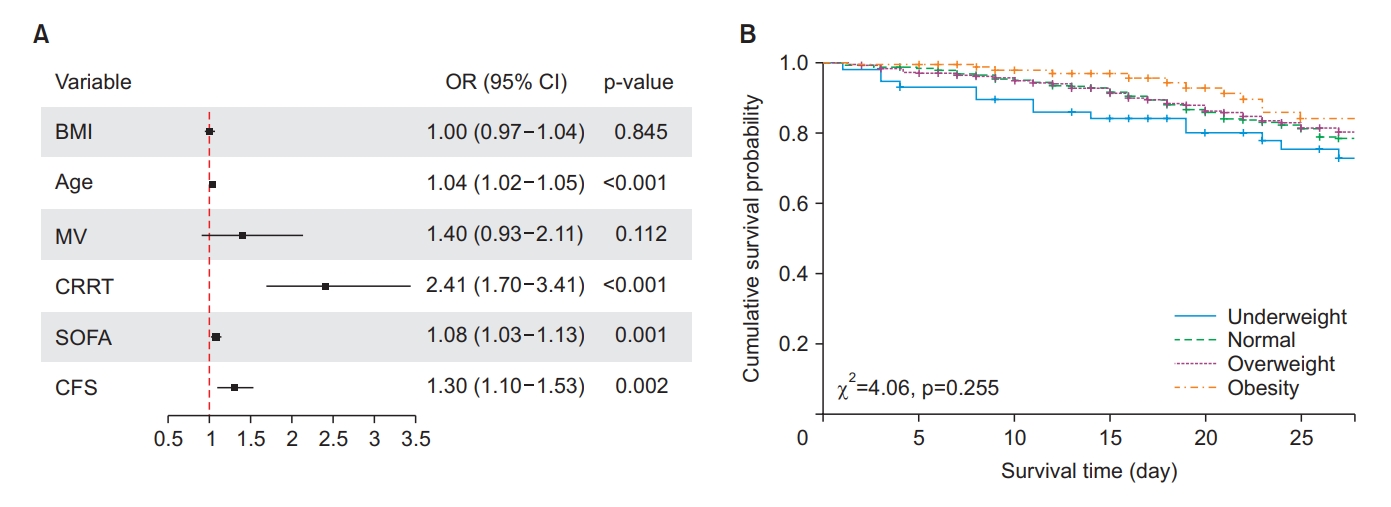
Table 1.
| Characteristic | Underweight (n=59) | Normal (n=541) | Overweight (n=389) | Obese (n=110) |
|---|---|---|---|---|
| Age, yr | 74.6±15.1* | 69.5±12.5 | 66.1±13.5§ | 59.6±16.5¶ |
| Male sex | 32 (54.2) | 321 (59.3) | 245 (63.0) | 68 (61.8) |
| BMI, kg/m2 | 17.0±1.9‡ | 22.6±1.7 | 27.0±1.3∥ | 32.8±3.2¶ |
| Smoking history | ||||
| Never smoker | 38 (64.4) | 370 (68.4) | 281 (72.2) | 74 (67.3) |
| Former smoker | 15 (25.4) | 91 (16.8) | 66 (17.0) | 16 (14.5) |
| Current smoker | 0 | 40 (7.4) | 20 (5.1) | 7 (6.4) |
| Unknown | 6 (10.2) | 40 (7.4) | 22 (5.7) | 13 (11.8) |
| Comorbidity | ||||
| Diabetes | 11 (18.6) | 180 (33.3) | 136 (35.0) | 41 (37.3) |
| Chronic neurologic disease | 20 (33.9) | 83 (15.3) | 38 (9.8) | 12 (10.9)§§ |
| Chronic lung disease | 9 (15.3) | 48 (8.9) | 21 (5.4) | 12 (10.9)†† |
| Cardiovascular disease | 14 (23.7) | 69 (12.8) | 37 (9.5) | 7 (6.4)†† |
| Chronic liver disease | 4 (6.8) | 13 (2.4) | 9 (2.3) | 3 (2.7) |
| Chronic kidney disease | 6 (10.2) | 37 (6.8) | 28 (7.2) | 6 (5.5) |
| Hypertension | 32 (54.2) | 274 (50.6) | 215 (55.3) | 66 (60.0) |
| Hematologic malignancy | 0 | 7 (1.3) | 7 (1.8) | 1 (0.9) |
| Solid malignant tumor | 8 (13.6) | 46 (8.5) | 18 (4.6) | 5 (4.5)†† |
| Connective tissue disease | 0 | 14 (2.6) | 1 (0.3) | 3 (2.7)†† |
| Immunocompromised | 2 (3.4) | 12 (2.2) | 9 (2.3) | 3 (2.7) |
| SOFA score | 6.0±3.8† | 4.6±3.0 | 4.4±2.9 | 3.8±2.6 |
| Lactate, mmol/L | 2.0±1.9 | 2.0±2.3 | 2.1±3.3 | 1.7±0.9 |
| Lowest ROX index** | 5.0 (0.1-7.3) | 4.8 (2.5-6.7) | 4.8 (0-7.6) | 4.7 (2.4-6.4) |
| Baseline CFS** | 5 (3-7)‡ | 3 (2-4) | 3 (2-3)§ | 3 (1.8-3)¶ |
| Source of infection | ‡‡ | |||
| Community | 45 (76.3) | 477 (88.2) | 354 (91.2) | 103 (93.6) |
| Nursing home | 3 (5.1) | 11 (2.0) | 3 (0.8) | 0 |
| Nursing hospital | 7 (11.9) | 27 (5.0) | 14 (3.6) | 1 (0.9) |
| Hospital | 4 (6.8) | 26 (4.8) | 17 (4.4) | 6 (5.5) |
| Treatment modalities | ||||
| High-flow nasal cannula | 24 (40.7) | 242 (44.7) | 160 (41.1) | 42 (38.2) |
| Mechanical ventilator | 33 (55.9) | 244 (45.1) | 186 (47.8) | 41 (37.3) |
| Mechanical ventilator and extracorporeal membrane oxygenation | 2 (3.4) | 55 (10.2) | 43 (11.1) | 27 (24.5)§§ |
| Prone position | 8 (13.5) | 105 (19.4) | 87 (22.4) | 27 (24.5)†† |
| Continuous renal replacement therapy | 5 (8.5) | 64 (11.8) | 35 (9.0) | 22 (20.0)†† |
Table 2.
| Variable | Underweight (n=59) | Normal (n=541) | Overweight (n=389) | Obese (n=110) |
|---|---|---|---|---|
| Death at 28 days | 14 (23.7) | 86 (15.9) | 57 (14.7) | 11 (10.0) |
| Hospital mortality | 18 (30.5) | 141 (26.1) | 100 (25.7) | 29 (26.4) |
| CFS at discharge‡ | 6 (3-7)* | 4 (3-6) | 3 (3-5)† | 3 (3-4.5) |
| Frail at discharge | 25 (61.0) | 146 (36.5) | 94 (32.6) | 20 (24.7)∥ |
| Tracheostomy | 16 (27.1) | 109 (20.1) | 57 (14.7) | 27 (24.5)§ |
| BSI | 15 (25.4) | 94 (17.4) | 72 (18.5) | 25 (22.7) |
| Duration of MV, day‡ | 15 (7-44) | 14 (8-32.3) | 14 (8-31.5) | 16.5 (8-38.5) |
| Hospital length of stay, day‡ | 26 (15-59) | 21 (14-36.8) | 21 (13-34.5) | 21 (14.8-36.5) |
Table 3.
| Characteristic |
Before propensity |
After propensity |
||
|---|---|---|---|---|
| Obese (n=499) | Nonobese (n=600) | Obese (n=429) | Nonobese (n=429) | |
| Age, yr | 65.0±14.3 | 69.7±13.0§ | 66.5±12.6 | 68.1±12.7 |
| Male sex | 313 (62.7) | 353 (58.8) | 263 (61.3) | 262 (61.1) |
| BMI, kg/m2 | 28.3±3.1 | 22.0±2.4§ | 28.0±2.9 | 22.1±2.2§ |
| Smoking history | ||||
| Never | 355 (71.1) | 408 (68.0) | 307 (71.6) | 286 (66.7) |
| Former | 82 (16.4) | 106 (17.7) | 72 (16.8) | 78 (18.2) |
| Current | 27 (5.4) | 40 (6.7) | 23 (5.4) | 30 (7.0) |
| Unknown | 35 (7.0) | 46 (7.7) | 27 (6.3) | 35 (8.2) |
| Comorbidity | ||||
| Diabetes | 177 (35.5) | 191 (31.8) | 152 (35.4) | 137 (31.9) |
| Chronic neurological disease | 50 (10.0) | 103 (17.2)‡ | 43 (10.0) | 57 (13.3) |
| Chronic lung disease | 33 (6.6) | 57 (9.5) | 30 (7.0) | 44 (10.3) |
| Cardiovascular disease | 44 (8.8) | 83 (13.8)† | 39 (9.1) | 52 (12.1) |
| Chronic liver disease | 12 (2.4) | 17 (2.8) | 10 (2.3) | 10 (2.3) |
| Chronic kidney disease | 34 (6.8) | 43 (7.2) | 30 (7.0) | 29 (6.8) |
| Hypertension | 281 (56.3) | 306 (51.0) | 247 (57.6) | 222 (51.7) |
| Hematological malignancy | 8 (1.6) | 7 (1.2) | 7 (1.6) | 5 (1.2) |
| Solid malignant tumors | 23 (4.6) | 54 (9.0)‡ | 21 (4.9) | 33 (7.7) |
| Connective tissue disease | 4 (0.8) | 14 (2.3)† | 4 (0.9) | 9 (2.1) |
| Immunocompromised | 12 (2.4) | 14 (2.3) | 10 (2.3) | 12 (2.8) |
| SOFA score | 4.3±2.9 | 4.7±3.1† | 4.3±2.9 | 4.6±3.1 |
| Lactate, mmol/L | 2.0±3.0 | 2.0±2.3 | 2.0±3.2 | 2.0±2.4 |
| Lowest ROX index* | 4.7 (0.1-7.4) | 4.8 (2.4-6.7) | 4.7 (0-7.4) | 4.9 (2.7-6.9) |
| Baseline CFS* | 3 (2-3) | 3 (2-4)§ | 3 (2-3) | 3 (2-4) |
| Source of infection | † | |||
| Community acquired | 458 (91.8) | 522 (87.0) | 398 (92.8) | 381 (88.8) |
| Nursing home | 3 (0.6) | 14 (2.3) | 3 (0.7) | 9 (2.1) |
| Nursing hospital | 15 (3.0) | 34 (5.7) | 11 (2.6) | 17 (4.0) |
| Hospital | 23 (4.6) | 30 (5.0) | 17 (4.0) | 22 (5.1) |
| Treatment modalities | ||||
| High-flow nasal cannular | 202 (40.5) | 266 (44.3) | 172 (40.1) | 195 (45.5) |
| Mechanical ventilator | 227 (45.5) | 277 (46.2) | 200 (46.6) | 182 (42.4) |
| Mechanical ventilator and ECMO | 70 (14.0) | 57 (9.5)† | 57 (13.3) | 52 (12.1) |
| Prone positioning | 114 (22.8) | 113 (18.8) | 98 (22.8) | 76 (17.7) |
| Continuous renal replacement therapy | 57 (11.4) | 69 (11.5) | 47 (11.0) | 53 (12.4) |
Table 4.
| Variable |
Before propensity |
After propensity |
||
|---|---|---|---|---|
| Obese (n=499) | Nonobese (n=600) | Obese (n=429) | Nonobese (n=429) | |
| Death at 28 days | 68 (13.6) | 100 (16.7) | 61 (14.2) | 74 (17.2) |
| Hospital mortality | 129 (25.9) | 159 (26.5) | 118 (27.5) | 104 (24.2) |
| CFS at discharge* | 3 (3-5) | 4 (3-6)† | 3 (3-5) | 4 (3-6)‡ |
| Frail at discharge | 114 (30.9) | 171 (38.8)† | 87 (28.1) | 152 (46.8)‡ |
| Tracheostomy | 84 (16.8) | 125 (20.8) | 72 (16.8) | 89 (20.7) |
| BSI | 97 (19.4) | 109 (18.2) | 84 (19.6) | 86 (20.0) |
| Duration of MV, day* | 14 (8-32.5) | 14 (8-33) | 14 (8-33) | 16 (8-33) |
| Hospital length of stay, day* | 21 (13-35) | 22 (14-39) | 20 (13-35) | 20 (14-37) |
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,020 View
- 69 Download
- ORCID iDs
-
Jeongsu Kim

https://orcid.org/0000-0001-6877-4289Hye Ju yeo

https://orcid.org/0000-0002-8403-5790Woo Hyun Cho

https://orcid.org/0000-0002-8299-8008 - Funding Information
-
Korean Academy of Tuberculosis and Respiratory Diseases
KATRD-S-2021-2Pusan National University
https://doi.org/10.13039/501100002543 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Supplement1
Supplement1 Print
Print Download Citation
Download Citation