 |
 |
| Tuberc Respir Dis > Volume 87(1); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Authors’ Contributions
Conceptualization: Park HJ. Methodology: Choi YJ, Byun MK, Park HJ. Formal analysis: Choi YJ, Park S. Data curation: Choi YJ, Lee MJ, Park S. Software: Choi YJ. Park S. Validation: Byun MK, Park HJ. Investigation: Park J, Park D, Kim SH, Kim Y, Lim SY, Yoo KH, Jung KS, Park HJ. Writing - original draft preparation: Choi YJ, Lee MJ. Writing - review and editing: Park HJ. Approval of final manuscript: all authors.
Funding
This research was supported by a fund from the research program of the Korea Medical Institute. In addition, this work was supported by the Research Program funded Korea National Institute of Health (Fund CODE 2016ER670100, 2016ER670101, 2016ER670102, 2018 ER67100, 2018ER67101, 2018ER67102, 2021ER120500, and 2021ER120501).
Fig. 1.

Fig. 2.

Fig. 3.
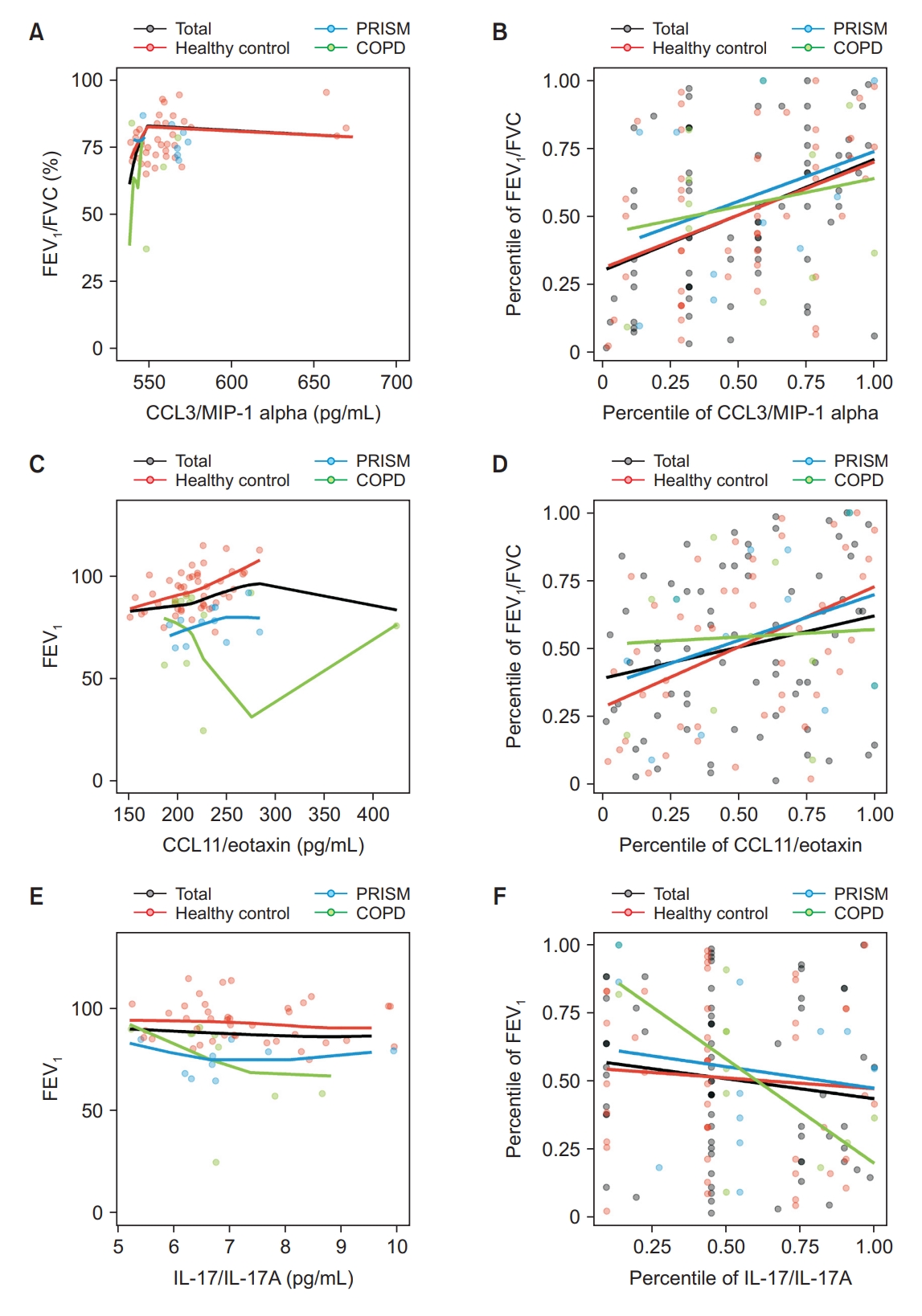
Fig. 4.

Table 1.
| Characteristic | Total (n=123) | Healthy control (n=81) | PRISM (n=21) | COPD (n=21) | p-value |
|---|---|---|---|---|---|
| Age, yr | 61.0 (52.0-67.0) | 62.0 (52.0-67.0) | 60.0 (58.0-67.0) | 61.0 (52.0-69.0) | 0.956 |
| Male sex | 94 (76.4) | 62 (76.5) | 16 (76.2) | 16 (76.2) | 0.999 |
| Weight, kg | 65.9±12.2 | 65.3±12.0 | 67.1±12.0 | 67.5±13.4 | 0.708 |
| Height, cm | 166.2±8.1 | 166.1±8.1 | 167.9±9.4 | 165.4±6.9 | 0.607 |
| Body mass index, kg/m2 | 23.8±3.5 | 23.6±3.3 | 23.8±3.8 | 24.6±4.1 | 0.545 |
| Smoking history | 0.895 | ||||
| Never smoker | 31 (25.4) | 21 (25.9) | 6 (30.0) | 4 (19.0) | |
| Former smoker | 52 (42.6) | 34 (42.0) | 9 (45.0) | 9 (42.9) | |
| Current smoker | 39 (32.0) | 26 (32.1) | 5 (25.0) | 8 (38.1) | |
| Symptoms (n=120) | |||||
| CAT score | 6.0 (4.0-11.0) | 5.0 (3.0-9.0) | 9.0 (5.0-14.0) | 12.5 (5.0-15.5) | 0.011* |
| SGRQ score (total) | 23.2 (8.3-35.6) | 19.9 (6.1-30.7) | 26.4 (15.3-44.3) | 32.7 (22.8-44.2) | 0.015* |
| Symptoms | 8.9 (3.4-16.9) | 5.4 (2.8-12.6) | 13.4 (6.6-26.4) | 13.2 (9.0-20.2) | 0.002* |
| Activity | 2.3 (0.0-9.6) | 2.1 (0.0-5.5) | 5.9 (2.1-17.1) | 5.9 (2.1-13.7) | 0.020* |
| Impacts | 7.6 (0.0-15.3) | 3.6 (0.0-14.9) | 14.9 (7.4-33.2) | 14.9 (7.4-23.1) | 0.002* |
| Pulmonary function test (n=117) | |||||
| FVC, L | 3.7 (3.2-4.2) | 3.8 (3.3-4.4) | 3.2 (2.7- 3.5) | 3.8 (3.2-4.3) | 0.005* |
| Predicted FVC, % | 87.0 (81.0-97.0) | 89.0 (82.5-97.5) | 71.0 (67.0-81.0) | 88.0 (84.5-99.5) | <0.001* |
| FEV1, L | 2.8±0.7 | 3.0±0.7 | 2.4±0.5 | 2.2±0.7 | <0.001* |
| Predicted FEV1, % | 3.2±0.6 | 94.0 (84.5-102.0) | 81.0 (57.5-88.0) | 78.5 (67.0-79.5) | <0.001* |
| FEV1/FVC, % | 76.0 (71.0-82.0) | 77.0 (72.0-82.5) | 75.5 (72.0-83.5) | 61.0 (46.0-67.5) | <0.001* |
| FEF25%-75%, L/sec | 2.1 (1.4-3.1) | 2.4 (1.9- 3.3) | 1.8 (1.4- 2.6) | 0.8 (0.6-1.3) | <0.001* |
| Predicted FEF25%-75%, % | 80.5 (58.0-104.0) | 94.0 (71.5-107.0) | 68.5 (55.5-86.0) | 36.0 (20.0-53.0) | <0.001* |
| Serologic test (n=123) | |||||
| White blood cell, 103/μL | 5.8 (4.8-6.9) | 5.4 (4.5-6.5) | 6.6 (5.4-8.2) | 6.5 (5.5-7.8) | 0.001* |
| Neutrophil, 103/μL | 3.3 (2.5-4.1) | 3.0 (2.2-3.5) | 4.3 (3.4-4.9) | 3.9 (2.9-4.3) | <0.001* |
| Neutrophil fraction, % | 56.7±10.2 | 54.4±9.7 | 64.2±9.8 | 58.3±8.6 | <0.001* |
| Lymphocyte, 103/μL | 1.8 (1.4-2.3) | 1.8 (1.4-2.3) | 1.6 (1.3-2.0) | 2.0 (1.6-2.5) | 0.118 |
| Lymphocyte fraction, % | 31.9±9.3 | 34.0±9.2 | 25.8±8.3 | 30.0±8.0 | 0.001* |
| Monocyte, 103/μL | 0.5±0.2 | 0.5±0.1 | 0.4±0.2 | 0.6±0.2 | 0.004* |
| Monocyte fraction, % | 7.9±2.2 | 8.2±2.1 | 6.7±2.5 | 8.2±2.0 | 0.021* |
| Eosinophil, 103/μL | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | 0.2 (0.1-0.2) | 0.2 (0.1-0.2) | 0.062 |
| Eosinophil fraction, % | 2.1 (1.4-3.6) | 2.1 (1.4-3.6) | 2.1 (1.5-3.6) | 2.1 (1.6-3.6) | 0.883 |
| Basophil, 103/μL | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.1) | 0.550 |
| Basophil fraction, % | 0.6 (0.4-0.8) | 0.7 (0.5-0.8) | 0.5 (0.4-0.7) | 0.7 (0.4-0.9) | 0.168 |
| Red blood cell, 106/μL | 4.5±0.5 | 4.5±0.5 | 4.5±0.6 | 4.6±0.4 | 0.625 |
| Hemoglobin, g/dL | 13.9±1.6 | 13.9±1.6 | 13.5±1.6 | 14.1±1.3 | 0.366 |
| Platelet, 103/μL | 235.0 (197.0-274.0) | 234.5 (195.5-271.0) | 232.0 (197.0-269.0) | 256.0 (212.0-321.0) | 0.356 |
Table 2.
Values are presented as median (interquartile range).
PRISM: preserved ratio impaired spirometry; COPD: chronic obstructive pulmonary disease; SD: standard deviation; ELISA: enzyme-linked immunosorbent assay; IL: interleukin; TNF-α: tumor necrosis factor α; TGF-β: transforming growth factor β; CCL: CC chemokine ligand; MIP: macrophage inflammatory protein; CXCL: CXC chemokine ligand; GRO: growth-regulated protein; CINC: cytokine-induced neutrophil chemoattractant; MMP: matrix metalloproteinase; GM-CSF: granulocyte-macrophage colony-stimulating factor; VEGF: vascular endothelial growth factor; PDGF: platelet derived growth factor; TSLP: thymic stromal lymphopoietin.
Table 3.
| Variable | Biomarkers |
Total |
Healthy |
COPD |
|||
|---|---|---|---|---|---|---|---|
| ρ | p-value | ρ | p-value | ρ | p-value | ||
| Demographic variables | |||||||
| Age, yr | TGF-β | -0.0834 | 0.5055 | -0.3458 | 0.0200* | 0.9524*,† | 0.0011*,† |
| Weight, kg | TNF-α | -0.0276 | 0.7873 | 0.0497 | 0.6829 | -0.5604† | 0.0298*,† |
| BMI, kg/m2 | VEGF | -0.2285 | 0.0553 | 0.0394 | 0.7904 | -0.7094† | 0.0145*,† |
| Symptomatic variables | |||||||
| CAT score | PDGF-AA | 0.1757 | 0.1371 | -0.0003 | 0.9986 | 0.5926*,† | 0.0423*,† |
| GM-CSF | -0.0522 | 0.6608 | 0.0476 | 0.7506 | -0.5777† | 0.0491*,† | |
| SGRQ score (total) | PDGF-AA | 0.0987 | 0.4095 | -0.1429 | 0.3378 | 0.6725*,† | 0.0166*,† |
| Symptoms | Granzyme B | 0.1583 | 0.1843 | 0.0176 | 0.9067 | 0.6078*,† | 0.0360*,† |
| Activity | PDGF-AA | -0.0792 | 0.5082 | -0.3051 | 0.0371* | 0.7176*,† | 0.0086*,† |
| IL-6 | 0.1985 | 0.0298* | 0.0187 | 0.8690 | 0.4671*,† | 0.0378*,† | |
| Impacts | PDGF-AA | 0.0148 | 0.9020 | -0.1663 | 0.2640 | 0.6151*,† | 0.0333*,† |
| Granzyme B | 0.1562 | 0.1902 | 0.0120 | 0.9360 | 0.6007*,† | 0.0389*,† | |
| Pulmonary function test | |||||||
| Predicted FEV1, % | CCL11/eotaxin | 0.2305 | 0.0567 | 0.4445*,† | 0.0017*,† | 0.0549 | 0.8726 |
| IL-17/IL-17A | -0.1409 | 0.2481 | -0.0765 | 0.6095 | -0.7024*,† | 0.0159*,† | |
| FEV1/FVC, % | IL-8 | 0.1395 | 0.1872 | 0.0366 | 0.7704 | 0.6227*,† | 0.0174*,† |
| CCL3/MIP-1 alpha | 0.4061*,† | 0.0005*,† | 0.3897 | 0.0068* | 0.2010 | 0.5534 | |
| White blood cell differential count | |||||||
| White blood cell, 103/μL | IL-5 | -0.0919 | 0.3486 | -0.0365 | 0.7606 | -0.6659*,† | 0.0035*,† |
| Neutrophil, 103/μL | IL-5 | -0.0955 | 0.3303 | 0.0122 | 0.9187 | -0.7080*,† | 0.0015*,† |
| Eosinophil, 103/μL | Pro-collagen I alpha 1 | 0.1030 | 0.3825 | 0.1850 | 0.2081 | 0.6735*,† | 0.0164*,† |
| MMP-12 | 0.2173 | 0.0629 | -0.0504 | 0.7338 | 0.7004*,† | 0.0112*,† | |
| Basophil, 103/μL | Pro-collagen I alpha 1 | 0.2151 | 0.0657 | 0.2166 | 0.1392 | 0.6295*,† | 0.0283*,† |
| Hemoglobin, g/dL | PDGF-AA | -0.0428 | 0.7176 | -0.1989 | 0.1754 | 0.6056*,† | 0.0369*,† |
COPD: chronic obstructive pulmonary disease; TGF-β: transforming growth factor β; TNF-α: tumor necrosis factor α; BMI: body mass index; VEGF: vascular endothelial growth factor; CAT: COPD assessment test; PDGF: platelet derived growth factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; SGRQ: St. George’s respiratory questionnaire; IL: interleukin; FEV1: forced expiratory volume in 1 second; CCL: CC chemokine ligand; MIP: macrophage inflammatory protein; FVC: forced vital capacity; MMP: matrix metalloproteinase.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,586 View
- 208 Download
- ORCID iDs
-
Yong Jun Choi

https://orcid.org/0000-0002-6114-2059Min Jae Lee

https://orcid.org/0009-0002-7480-4071Hye Jung Park

https://orcid.org/0000-0002-1862-1003 - Funding Information
-
Korea Medical Institute
Korea National Institute of Health
https://doi.org/10.13039/501100003653
2016ER670100
2016ER670101
2016ER670102
2018 ER67100
2018ER67101
2018ER67102
2021ER120500
2021ER120501 - Related articles
-
Two Cases of Inflammatory Pseudotumor in Respiratory System.1999 March;46(3)


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



