Problems in the Pathologic Diagnosis of Suspected Lung Cancer
Article information
Abstract
Since the introduction of low-dose computed tomography (CT) screening for patients at high risk of lung cancer, the detection rate of suspicious lung cancer has increased. In addition, there have been many advances in therapeutics targeting oncogenic drivers in non-small cell lung cancer. Therefore, accurate pathological diagnosis of lung cancer, including molecular diagnosis, is increasingly important. This review examines the problems in the pathological diagnosis of suspected lung cancer. For successful pathological diagnosis of lung cancer, clinicians should determine the appropriate modality of the diagnostic procedure, considering individual patient characteristics, CT findings, and the possibility of complications. Furthermore, clinicians should make efforts to obtain a sufficient amount of tissue sample using non- or less-invasive procedures for pathological diagnosis and biomarker analysis.
Introduction
Lung cancer has become a major cause of cancer mortality worldwide [1]. Although the number of smokers in South Korea has been continuously decreasing, the incidence of lung cancer in non-smokers and women is increasing. Additionally, these lung cancers have different pathologies and genetic alterations than lung cancer associated with smoking and have a higher proportion of known oncogenic drivers [2-4]. Currently, various targeted agents for these driver mutations, such as epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase fusion, are available for patients with advanced non-small cell lung cancer (NSCLC). With the recent development of new drugs targeting v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations, rearranged during transfection (RET) rearrangements, mesenchymal-epithelial transition factor (MET) exon 14 skipping mutations, and kirsten rat sarcoma virus (KRAS) G12C mutations, NSCLC patients have opportunities to receive personalized treatment based on the results of molecular tests [5]. However, appropriate tissues must be collected for accurate testing of predictive biomarkers to determine which patients are best suited for these targeted agents.
The detection rate of lung lesions suspicious for lung cancer has improved since the introduction of low-dose computed tomography (CT) screening for high risk patients [6]. However, as most of the lung lesions found on low-dose CT screening are pathologically benign, it is important to obtain a pathological diagnosis for lung lesions with positive screening results [7]. Three methods have been used for the pathological diagnosis of lung lesions: (1) thoracoscopic wedge resection; (2) transthoracic needle biopsy (TTNB); and (3) bronchoscopy, such as radial probe endobronchial ultrasonography (EBUS), virtual bronchoscopy navigation (VBN), and electromagnetic navigation bronchoscopy (ENB). Due to the surgical risk of thoracoscopy, the importance of non-surgical biopsy methods, such as TTNB or bronchoscopy, is increasing [8]. However, determining which modality is best for each patient can be confusing. In this review, we examine the potential concerns and problems of pathological diagnosis of suspected lung cancer and molecular diagnoses in advanced stages.
Problems in the Pathological Diagnosis of Early Lung Cancer
1. Comparison of biopsy modalities for the diagnosis of lung cancer
Among the three methods for pathological diagnosis of lung cancer, thoracoscopic wedge resection is the most accurate, but it has a relatively high complication rate [9]. In a recent study by Nunez et al. [10], thoracic surgery as a biopsy modality was significantly associated with major complications such as acute respiratory failure (4.5%) and prolonged mechanical ventilation (2.6%) and intermediate complications including cardiac arrhythmia requiring medical attention (14.0%), pain (9.9%), infection requiring antibiotics (including pneumonia) (8.8%), pleural effusion (5.9%), and hemorrhage or hemoptysis (4.3%) compared with non-surgical procedures (odds ratio, 7.70; 95% confidence interval [CI], 5.48 to 10.81). Therefore, the importance of advances in non-surgical approaches such as radial probe EBUS, VBN, and ENB during peripheral bronchoscopy, in addition to conventional TTNB, is rising, especially in elderly patients and patients with comorbidities [9]. Because it is associated with a lower risk of complications, non-surgical biopsy should be considered when evaluating lung nodules with a low-to-moderate risk of malignancy (10% to 60%), and surgical biopsy should be reserved for lung nodules with a high probability of malignancy (>65%) [9]. Clinicians should be aware of the possibility of complications after biopsy as they can outweigh the benefits of diagnosis of lung cancer. The three biopsy modalities are compared in Table 1 [9,11-15].
2. Factors related to the performance of TTNB
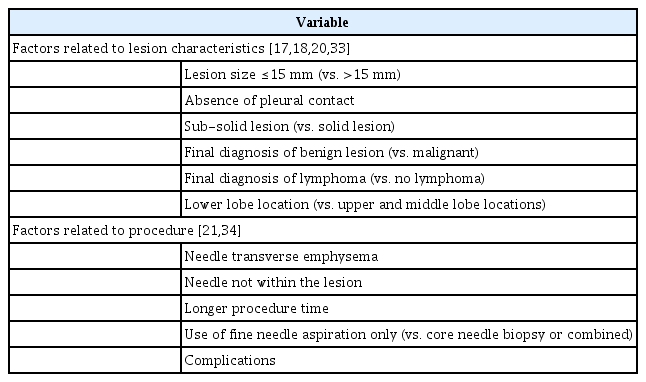
CT-guided TTNB has a high diagnostic accuracy, with a sensitivity and specificity of 90% and 95%, respectively [11]. However, a recent meta-analysis showed that the non-diagnostic result rate of TTNB is 6.8% (95% CI, 6.0 to 7.6; I2=0.91), which may hamper the clinical diagnosis of lung cancer and needs further investigation [16]. Table 2 describes the factors related to a non-diagnostic result in TTNB, which include lesion size ≤15 mm, absence of pleural contact, sub-solid lesion, final diagnosis of benign lesion or lymphoma, lower lobe location, needle transverse emphysema, needle not within the lesion, longer procedure time, use of fine needle aspiration only, and complications [17-20]. Although TTNB is considered safe, procedure-related complications, such as iatrogenic pneumothorax and bleeding, can occur [21]. Table 3 describes the factors related to complications after TTNB [21-26]. It is noteworthy that comorbidities such as chronic obstructive pulmonary disease (COPD) and the presence of honeycombing on CT are significantly associated with complications. As the prevalence of COPD (76%) [27] and interstitial lung disease (ILD) (worldwide, 2% to 24%; Korea, 2%; Japan, 5% to 24%; European countries, 2% to 5%) [28,29] are not low in patients with lung cancer, clinicians should be aware of these conditions when determining the biopsy method. In summary, TTNB is recommended for lesions in the peripheral area of the lung (especially those with pleural contact) but should be performed with caution or contraindicated for lesions in the central area or lower lobe and in patients with comorbidities such as COPD, pleural effusion, and ILD (with honeycombing).
3. Factors related to the performance of peripheral bronchoscopy
Although conventional bronchoscopy is safe, the diagnostic yield of bronchoscopy for peripheral lung lesions (approximately 30%) is markedly lower than that of TTNB [9]. Technological advances in bronchoscopy have overcome this issue, such as the addition of radial probe EBUS, VBN, and ENB. The pooled diagnostic yield of transbronchial biopsy (TBB) combined with radial probe EBUS is 71% to 73% [12-14]. In a meta-analysis by Ali et al. [13], lesion size (≤20 mm vs. >20 mm: 60.5% vs. 75.7%, p<0.001), final diagnosis (malignant vs. benign: 72.4% vs. 60.2%, p=0.018), position of radial EBUS (within vs. adjacent: 78.7% vs. 52.0%, p<0.001), and bronchus sign (yes vs. no: 76.5% vs. 52.4%, p=0.008) were significantly associated with the diagnostic yield of TBB with radial EBUS. In addition, the VBN system significantly improves the diagnostic yield of TBB compared with non-VBN (80.4% vs. 67%, p=0.032) when combined with radial EBUS and fluoroscopy [30]. With regard to ENB, the pooled diagnostic yield of TBB is 72% (95% CI, 66 to 76), which is increased to 80% (95% CI, 74 to 83) when combined with both radial probe EBUS and ENB [15]. Recently, ultrathin bronchoscopy (UTB) (external diameter, ≤3.5 mm) has been developed for the diagnosis of lung cancer [31]. UTB showed a promising result compared to thin bronchoscopy (external diameter, 4.0 or 4.2 mm) in lesions ≤20 mm (62.7% vs. 51.5%, p=0.027) and those in the peripheral area (69.3% vs. 55.6%, p=0.019) among patients who underwent TBB combined with radial EBUS and VBN [32]. Hence, TBB is recommended as a priority for patients with a positive bronchus sign, and multimodal approaches could enhance the diagnostic yield of TBB. Furthermore, TBB can be a reasonable alternative modality in patients with a high risk of complications during TTNB. However, TBB using these techniques might be limited in the real-world setting because of the high cost.
4. Non-diagnostic results in non-surgical biopsy
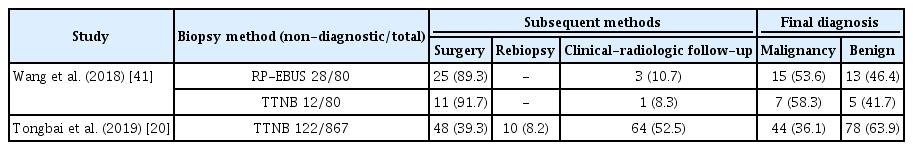
Unlike samples obtained after thoracoscopic wedge resection, there is a possibility of non-diagnostic results after non-surgical biopsy, where diagnosis is based on lesion characteristics such as size, location, distance from the pleura, and necrotic proportions within lesions [16,17]. Most studies on non-diagnostic results have focused on TTNB, which had a 6.8% non-diagnostic result rate in a meta-analysis [16]. However, due to heterogeneity of the definition of non-diagnostic results and prevalence of malignancy, non-diagnostic result rates range from 0.6% to 35% [17,18,33-40]. For accurate diagnosis in such patients, further investigations are required, such as surgical resection, repeated non-surgical biopsy, or clinical and radiological follow-up for at least 2 years [20]. Table 4 shows an example of non-diagnostic results with final diagnoses after further investigations [20,41]. Clinicians should be aware of the potential for non-diagnostic results during non-surgical biopsy, and possibilities of hidden malignancy should be considered.
Problems in the Molecular Diagnosis of Advanced Lung Cancer
1. Identification of driver mutations in lung cancer
As the development of targeted agents and immunotherapy advances, various diagnostic modalities are needed to determine which patients would benefit from which treatments. This means that respiratory doctors should secure more tissue volume during biopsy [42]. If the driver mutation cannot be confirmed due to the spatiotemporal heterogeneity of the tumor or inadequate tissue sample, the patient may be administered inappropriate treatment, which can seriously affect the clinical course [43]. Lindeman et al. [44] reported that the concordance of EGFR mutation tests between the primary tumor and metastatic lesions was 94%, which could be interpreted as the possibility that some EGFR mutation tests are false-negative. In addition, Kim et al. [45] reported that the concordance of EGFR mutation results between small biopsy samples and surgical specimens was 97% (concordant in 88 of 91 study patients). However, three patients with discordant EGFR mutation test results had EGFR mutation-positive surgical specimens, despite EGFR mutation being undetected in small biopsy samples. Therefore, when there is ambiguity in the results of mutational analysis (e.g., a NSCLC patient with a certain driver mutation shows an atypical clinical course or an NSCLC patient who is relatively young or has no smoking history does not have a driver mutation), rebiopsy should be considered for accurate evaluation of somatic mutations and to update treatment as necessary.
2. Next-generation sequencing
Next-generation sequencing (NGS), which enables simultaneous multigene testing, has become increasingly important in the diagnosis of NSCLC. Although NGS is an excellent method in precision medicine, its major limitation is that a sufficient amount of high-quality tissue must be collected to perform NGS in clinical practice, increasing the burden on respiratory physicians. Murakami et al. [46] reported successful sequencing rates of 57% to 97% for samples retrieved using TBB with radial probe EBUS and 63% to 100% for endobronchial biopsy. In their analyses, a suitable sample for NGS had a tumor concentration ≥30% and tissue surface area ≥1 mm2, regardless of the bronchoscopy method. Moreover, Kage et al. [47] found that 80% to 100% of TTNB samples, 82% to 100% of EBUS-guided transbronchial needle aspiration samples, and 73% to 82% of TBB samples were successfully used for DNA or RNA NGS assays.
Cryobiopsy is a recently introduced, novel TBB method that can be used to collect a large amount of tissue to overcome the limitations of conventional TBB. Udagawa et al. [48] reported that the specimen size and amounts of DNA and RNA extracted from samples obtained using cryobiopsy were significantly larger than those taken from samples obtained using forceps biopsy (median sample size: 15 mm2 vs. 2 mm2, p<0.01; median DNA amount: 1.60 µg vs. 0.58 µg, p<0.01; and median RNA amount: 0.62 µg vs. 0.17 µg, p<0.01). However, there are concerns about the complications of cryobiopsy, such as significant bleeding. Matsumoto et al. [49] recently reported that the diagnostic yield of conventional TBB and sequential cryobiopsy combined with radial probe EBUS, VBN, and fluoroscopy was 90% in patients with peripheral lung lesions, and only 1.2% of patients developed severe hemorrhage and 0.8% developed pneumothorax. If patients are carefully selected based on patient characteristics and CT findings, cryobiopsy will be an effective biopsy method to retrieve samples for NGS without increasing serious morbidity and mortality [50,51].
Conclusion
For successful pathological diagnosis of lung cancer, clinicians should determine the appropriate modality, considering individual patient characteristics, CT findings of the lesion, and possibility of complications. Furthermore, clinicians should try to obtain large tissue samples with good quality for the pathological diagnosis and biomarker analysis in the era of precision medicine.
Notes
Authors’ Contributions
Conceptualization: Eom JS. Writing - original draft preparation: Kim SH, Eom JS. Writing - review and editing: Kim SH, Kim MH, Lee MK, Eom JS. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a clinical research grant from Pusan National University Hospital in 2023 and also by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2022R1F1A1074117).