 |
 |
| Tuberc Respir Dis > Volume 86(3); 2023 > Article |
|
Abstract
Bronchiectasis, which is characterized by irreversibly damaged and dilated bronchi, causes significant symptoms, poor quality of life, and increased economic burden and mortality rates. Despite its increasing prevalence and clinical significance, bronchiectasis was previously regarded as an orphan disease, and ideal treatment of this disease has been poorly understood. The European Respiratory Society and British Thoracic Society have recently published guidelines to assist physicians in the clinical field. Guidelines and reports suggest comprehensive management that includes both non-pharmacological and pharmacological treatment. Physiotherapy and pulmonary rehabilitation are two of the most important non-pharmacologic therapies in bronchiectasis patients; long-term inhaled antibiotics and macrolide therapy have gained significant evidence in reducing exacerbation risk in frequent exacerbators. In this review, we summarize recent updates on bronchiectasis treatment to prevent exacerbation and manage clinical deterioration.
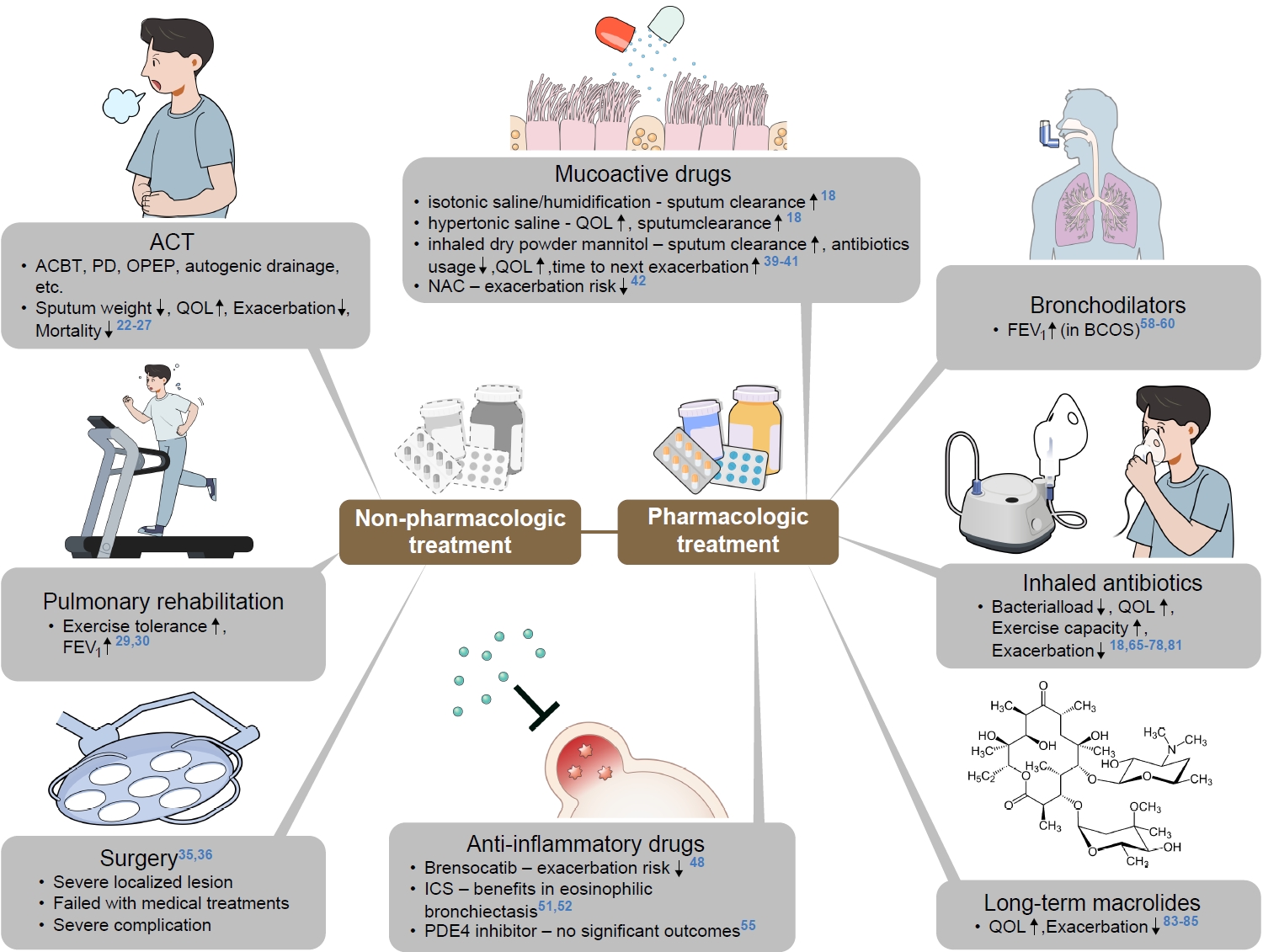
Bronchiectasis is a chronic respiratory disease that is characterized by irreversibly damaged and dilated bronchi, and is mainly associated with recurrent airway infection and inflammation [1,2]. It was previously regarded as an orphan disease with prevalence of less than 50 per 100,000 individuals [3]; however, recent studies have shown that the prevalence of bronchiectasis has increased to (200 to 600) per 100,000 worldwide [4-9]. It was also shown The presence of bronchiectasis was also shown to be associated with increased economic burden, hospital admission, and mortality rate [9-14]. In particular, the exacerbation of bronchiectasis is a hazardous condition, and causes poor quality of life and increased mortality [15-17]. Those who have frequent exacerbation history are at risk of future exacerbation, and those frequent exacerbators show poorer quality of life, and higher rates of hospitalization and mortality [16]. Although awareness of this disease is increasing, a consensus on ideal management has not yet been reached. This review summarizes recent updates of bronchiectasis with a focus on exacerbation prevention and management (Figure 1).
Airway clearance technique (ACT) is a technique that aims to remove mucus secretion with effective expectoration, and to control the impact of cough on the quality of life [18,19]. It consists of postural drainage, effective breathing techniques, such as the active cycle of breathing technique (ACBT) and autogenic drainage, and devices that facilitate the expiratory process or produce chest wall oscillation may be used [19]. Good performance of ACT has been believed to improve sputum clearance and elevate the quality of life in bronchiectasis patients; however, there is a paucity of randomized controlled studies that investigate the effect of ACT on bronchiectasis patients [18-20]. Among various ACTs, ACBT is the most commonly used in bronchiectasis patients [21]. It has been shown to be as effective as oscillating positive expiratory pressure (OPEP) in sputum weight, quality of life, and lung function in bronchiectasis patients [22-24]. OPEP devices, such as Flutter and Acapella devices, have been shown to be effective in sputum expectoration and quality of life in a systemic review that included seven studies (n=146 patients) [25]. However, there were no significant differences in sputum expectoration, lung function, gas exchange, or symptoms change, compared to other ACTs. Munoz et al. [26]’s randomized controlled study studied the promising effect of slow expiration with the glottis opened in the lateral posture (ELTGOL) technique in bronchiectasis patients. After 1 year of twice-daily performance of the ELTGOL technique, bronchiectasis patients showed facilitated sputum removal, decreased exacerbation frequency, improved quality of life, and decreased cough score.
Huang et al. [27] used data from a multicenter bronchiectasis cohort in Taiwan to evaluate the comorbidities and effect of ACT performance on mortality rates in bronchiectasis with severe exacerbation history. In the study, patients with multi-morbidities (bronchiectasis aetiology comorbidity index ≥6) showed higher 1 year mortality rate, compared to those with lesser comorbidities (p<0.01) [27]. In both comorbidity groups, those who performed ACT showed decreased 1 year mortality. In addition, multivariate analysis presented that the performance of ACT was associated with lower mortality risk in overall patients (hazard ratio [HR], 0.50; p=0.010).
Also, bronchoscopic-ACT (B−ACT), which refers to bronchial toileting and bronchoalveolar lavage with bronchoscopy, has shown promising outcome in bronchiectasis patients with acute exacerbation [28]. Liu et al. [28] reported the result of a randomized controlled study aimed to evaluate the efficacy and safety of B− ACT in those with moderate-to-severe exacerbation of bronchiectasis. The B−ACT group showed delayed time to first exacerbation (HR, 0.57; p=0.024) compared to control group; those with frequent exacerbation and greater symptoms showed more prominent results. There were no adverse events of concern in the B−ACT group. However, the data has the limitation of being a preliminary study, and further larger studies are needed.
In the 2019 British Thoracic Society (BTS) guideline, pulmonary rehabilitation (PR) was recommended in those with functional limitation caused by breathlessness (modified Medical Research Council [mMRC] ≥1). They recommended the use of inspiratory muscle training, in addition to conventional PR [18]. Recently, two meta-analyses were published that aimed to evaluate the efficacy of PR in bronchiectasis patients. Ora et al. [29] analyzed the effectiveness of PR on pulmonary function and exercise tolerance in non-cystic bronchiectasis patients. There was no significant improvement in forced expiratory volume in 1 second (FEV1) in the PR group, compared to control group. However, exercise tolerance was improved in the PR group, as assessed by the incremental shuttle walk test (ISWT) (mean difference=68.85 m; p<0.001), and 6-minute walk test (mean difference=37.7 m; p<0.001). While Yang et al. [30]’s meta-analysis showed similar result on exercise tolerance in comparison between the PR and non-PR group, FEV1 was higher in the PR group compared to the non-PR group (median difference=0.08 L; p<0.001). St. George’s Respiratory Questionnaire (SGRQ) and Leicester Cough Questionnaire score were not improved by PR. These two meta-analyses insist that PR in bronchiectasis patients plays a clear role in the improvement in exercise tolerance, and has a potential role in lung function improvements.
Recently, a single center randomized controlled study investigated the effect of home-based PR on bronchiectasis patients, compared to those with general advice on physical activity and education [31]. After 8 weeks, the home-based PR group showed improved exercise capacity, dyspnea scale, and physical activity, compared to control group.
To elucidate those who have more effective outcomes in PR performance, Candemir et al. [32] reported a retrospective study to identify factors that are associated with improvement in bronchiectasis patients who received a multidisciplinary PR program. In the study, mMRC score, SGRQ, ISWT, endurance shuttle walk test, and Hospital Anxiety and Depression scores were improved after undergoing the PR program, regardless of sex, etiology, smoking status, or number of hospitalizations. The younger age group showed more improvement in quality of life, but no associations with exercise capacity, or anxiety and depression, were observed. Those with low baseline exercise capacity were more likely to show improvement in exercise capacity. Improvement of quality of life was more prominent in those with young age, less baseline symptoms, better quality of life, and higher baseline FEV1. Chalmers et al. [33] studied the effect of PR following the exacerbation of bronchiectasis. Unlike chronic obstructive pulmonary disease (COPD), there were no beneficial effect of PR in terms of exercise capacity, time to the next exacerbation, quality of life, lung function, and cough impact, compared to the standard care group [33]. These results suggest that PR may be more beneficial in those without recent exacerbation, although a relatively small number of patients were included in the study. Finding appropriate candidates who would benefit from PR is important in the era of personalized medicine, and more studies are needed in the future.
In a recent prospective observational study, the association between level of physical activity and clinical outcomes was evaluated in bronchiectasis patients [34]. Those who walk ≤6,290 steps or spend ≥7.8 hours per day of sedentary behavior showed increased risk of hospitalization in the following year. In particular, ≥7.8 hours per day of sedentary behavior was independently associated with a 5.9-fold increased risk of hospitalization during 1-year follow-up. These data indicate that not only are patients’ education and rehabilitation important, but also their actual physical activity in life makes remarkable outcomes in bronchiectasis patients.
The role of surgery is limited in bronchiectasis patients. Indications include patients with severe localized lesion who failed with all medical treatments, or those with severe complications, such as life-threatening hemoptysis or recurrent pneumonia [35,36]. Recently, Selman et al. [36] published a report that shows 5-year experiences of surgically treated bronchiectasis in Central Europe. The main indications of surgical resection were failure of best medical therapy (26%), and massive hemoptysis (12%). Of all, 67% of patients showed excellent satisfaction, 30% reported good satisfaction, and only 3% reported no benefit from the surgery. Despite the favorable outcome in surgery, clinicians should be aware of possible complications of surgery; 15% of patients showed atelectasis, 21% presented persistent air leak >7 days, 3% showed empyema, 21% showed pleural effusion, and 3% were deceased. Careful selection of patients to undergo surgical resection is crucial.
Mucoactive drugs are medications that directly impact the mucus in the bronchus. They are roughly categorized as expectorants, mucolytics, mucokinetics, and mucoregulators by their mechanism of actions, although some agents function with multiple mechanisms [18]. Research has focused on mucolytics (DNAse), expectorants (humidification or isotonic saline), hypertonic saline, and mannitol. In the European Respiratory Society and BTS guidelines, the authors recommended not to use recombinant human DNase, as it was reported to increase exacerbation risk, and worsen lung function in the previous data [18,19,37]. There were some small studies that showed potential benefit of humidification and isotonic saline on sputum clearance, and hypertonic saline on quality of life and sputum clearance in bronchiectasis patients; yet further large studies are needed [18]. A phase 3 randomized study has shown that inhaled dry powder mannitol improved 24 hours sputum weight and decreased antibiotics usage compared to placebo group, in bronchiectasis patients [38]. Another phase 3 randomized study on bronchiectasis was performed to evaluate the benefits in exacerbation risk between inhaled mannitol 400 mg and low-dose mannitol usage group [39]. Although the exacerbation rate, which was the primary endpoint, did not meet statistically significance, time to first exacerbation was delayed in the mannitol group (HR, 0.79; p=0.22), while SGRQ score was improved (-2.4 units, p=0.046). Recently, post hoc analysis of this study was performed, and exacerbation rate evaluated according to baseline symptom burden using SGRQ score [40]. In this study, those with high symptom burden showed delayed time to first exacerbation in the mannitol treatment group (relative risk [RR], 0.56; p<0.01), but no significant differences were shown in the low symptom burden group. These results indicate that the mannitol inhalation may only show benefit in the highly symptomatic bronchiectasis group. Several side effects, including cough, dyspnea, sore throat, nasopharyngitis, and headache, may be possible, and when using inhalant drugs, clinicians should be cautious [39,41].
In COPD patients, mucoregulators, including N-acetylcysteine (NAC) and erdosteine, were recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline, as these drugs have shown promising outcome in reducing exacerbation risk (evidence B). However, there have been only small and inconclusive data regarding the benefits of mucoregulators in bronchiectasis patients. Recently, a randomized controlled study (The Effect of NAC on Exacerbations of Bronchiectasis [BENE]) has presented that the NAC group showed decreased incidence of exacerbation in 12-month follow-up compared to the on-demand group (1.31 vs. 1.98) per patient-year (RR, 0.41; p=0.001) [42]. Sputum volume was also remarkably decreased, and quality of life was enhanced in the NAC group, compared to the on-demand group. Further multicenter randomized controlled study and meta-analysis are ongoing to reveal the effect of NAC in bronchiectasis patients [43,44].
The pathogenesis of bronchiectasis has been described as a vicious cycle of airway dysfunction that disrupts normal host defense, causing vulnerability to chronic infection, leading to provocation of host inflammatory response, resulting in airway injury/remodeling that aggravates the structural disease of bronchiectasis [45]. Although bronchiectasis is a heterogeneous disease, neutrophilic inflammation has been considered the key factor of the vicious cycle of lung injury [46].
Recent molecular studies have reinforced the importance of neutrophilic inflammation in the clinical outcomes of bronchiectasis patients. Neutrophil elastase (NE) is a protease that is accumulated in azurophilic granules and released during degranulation, neutrophil extracellular trap (NET) formation, or cell death. In a single center prospective cohort study, sputum NE has been shown to be associated with elevated exacerbation frequency (p<0.0001), FEV1 decline rate (β coefficient=−0.139, p=0.001), and presented to be a good discriminator for time to next exacerbation, hospitalization, and all-cause mortality (p<0.0001, p<0.0001, p<0.0001, respectively) in 3-year follow-up period [47]. In addition, NET formation is a dynamic process in which neutrophils actively release a network of extracellular fibers made up of chromatic DNA, histones, and bactericidal proteins to trap and neutralize pathogens. Recent multicohort observational study has identified that sputum NETs were associated with bronchiectasis severity index, quality of life, risk of hospitalization, and mortality in bronchiectasis patients. Also, a decrease in NETs was observed in patients who showed a favorable treatment response to systemic antibiotics and macrolides during acute exacerbation.
Multiple studies have been performed to evaluate the benefits of various anti-inflammatory drugs, according to the hypothesis that neutrophilic inflammation is attenuated with anti-inflammatory drugs, which result in improved clinical outcomes in bronchiectasis. Brensocatib is an oral reversible inhibitor of dipeptiyl peptidase 1, which is an enzyme that is associated with the activation of neutrophil serine protease, which enables the packaging of active enzymes into granules before neutrophil is released into circulation [48]. Chalmers et al. [48] reported a phase 2 randomized, double-blind, placebo-controlled trial that investigated the potential benefits of brensocatib in bronchiectasis patients with ≥2 exacerbation per year, and who had chronic purulent sputum. After 24 weeks of treatment, the brensocatib group showed delayed time to first exacerbation (10 mg vs. placebo, p=0.03; 25 mg vs. placebo, p=0.03) and decreased exacerbation risk (10 mg vs. placebo: HR, 0.58; 95% confidence interval [CI], 0.35 to 0.95) (25 mg vs. placebo: HR, 0.62; 95% CI, 0.38 to 0.99), compared to the placebo group. However, there were some issues that periodontal disease and skin hyperkeratosis was more frequent in the brensocatib group. Further phase 3 trial is ongoing, and estimated to be completed in March, 2024 (NCT04594369).
Inhaled corticosteroids (ICSs) may reduce sputum volume, but increased systemic adverse effect should be considered. Hakansson et al. [49] recently analyzed all-cause mortality associated with ICS use in bronchiectatic patients. High-dose ICS use was associated with increased mortality after adjustment for age, sex, FEV1, and concomitant asthma or COPD (HR, 4.93; p=0.003). Also, patients who used ICS showed more pseudomonas colonization and previous severe exacerbations, compared to those who did not.
Although bronchiectasis was believed to be associated with neutrophilic inflammation, a European multicohort study has identified that 22.6% of bronchiectasis patients were eosinophilic (blood eosinophil counts ≥300 cells/μL) [50]. High eosinophil count (≥300 cells/μL) was associated with shorter time to next exacerbation, compared to low eosinophil count (<100 cells/μL) in bronchiectasis patients (HR, 3.99; p<0.0001). Recent studies have investigated the potential benefits of ICS in eosinophilic bronchiectasis [51,52]. In a pooled post hoc analysis of two randomized clinical studies, the number of exacerbations and hospitalizations were reduced in the use of ICS for those with eosinophilia, compared to those without (exacerbations: 1 [0−2] vs. 0 [0−1], p=0.029; hospitalization: 0 [0−1] vs. 0 [0−0], p=0.039) [52]. Also, a research letter has reported that 6-month treatment with ICS has shown significant improvement in quality of life in bronchiectasis with peripheral eosinophilia (≥3%) [51]. Further studies are needed for the precise indication for ICS use in bronchiectasis.
For eosinophilic bronchiectasis, there has been an interesting case series that showed the potential benefit of anti-interleukin 5 (IL5) and anti-IL5Rα [53]. Rademacher et al. [53] recruited 21 bronchiectasis patients with frequent exacerbation, chronic airflow limitation, decreased quality of life, and persistent peripheral eosinophilia (≥300 cells/uL), despite optimal therapy. Twelve patients were treated with mepolizumab, and nine were on benralizumab. After 6 months of therapy, the treatment group showed increased FEV1, and decreased exacerbation rates and dyspnea score. Also, peripheral eosinophil count was decreased, while 24 hours sputum volume was decreased. All patients (n=14) who were on long-term oral corticosteroids (OCS) showed OCS-sparing effect.
Phosphodiesterase 4 (PDE4) inhibitor is a potent anti-inflammatory drug that significantly reduces exacerbation risk in severe COPD with chronic bronchitis phenotype and frequent exacerbators [54]. Juthong and Panyarath [55] recently published a double-blinded, randomized, placebo-controlled trial that evaluated the efficacy of PDE4 inhibitor in bronchiectasis patients with frequent exacerbation. Although there were limitations of a small study population (n=30), it was the first trial that evaluated the efficacy of PDE4 inhibitor on exacerbation risk or lung function in bronchiectasis patients. There were no statistical differences in exacerbation rate or lung function between the user group and placebo group, while there were some concerns about the increased adverse effects related to PDE4 inhibitor use (mainly gastrointestinal side effects). Still, there is a lack of evidence to routinely use PDE4 inhibitor in bronchiectasis patients, and further studies are ongoing (NCT04322929).
COPD and bronchiectasis frequently co-exist in patients, so that 54.3% of COPD patients have reported to have bronchiectatic features [56]. Among bronchiectasis patients, the prevalence of COPD ranged (27% to 69%) in previous studies [9,56,57]. These bronchiectasis and COPD overlap syndrome (BCOS) patients have shown to have poorer outcomes, including frequent exacerbation, lower lung function, increased mucus production, and increased mortality [11,12,56]. There have been recent interests on this phenotype, and efforts to assess their clinical features and optimal treatments are ongoing.
In the BTS guideline, bronchodilators in bronchiectasis were only recommended in those with co-existing COPD or asthma [56]. Also, the guideline suggested that a long-acting bronchodilator may be beneficial in those with severe breathlessness, although there was limited evidence. Previous studies have shown that the use of bronchodilator in bronchiectasis patients with airflow limitation leads to the improvement of lung function [58,59]. Jayaram et al. [60] recently published a randomized, double-blind, two-period crossover study that aimed to assess the efficacy of using tiotropium in bronchiectasis with airflow limitation. Using tiotropium had no benefits on exacerbation risk, exercise capacity, quality of life, or symptoms. However, the tiotropium group showed increased FEV1 (58 mL, p=0.002) and forced vital capacity (78 mL, p=0.005) compared to the placebo group, in 6 months of follow-up. Using bronchodilators in selected bronchiectasis patients may be beneficial, and further studies are needed to determine which patients to use, with regard to patient-tailored therapy.
The pathogenesis of bronchiectasis was described by Flume et al. [45] as interconnection and vicious cycle of infection, inflammatory response, airway dysfunction, and structural change. Chronic airway infection is a treatable trait of bronchiectasis that if properly managed, has the potential to have a positive effect on clinical outcomes [61]. In particular, for those with chronic pseudomonas colonization, it is associated with poor quality of life and lung function, and increased exacerbations, hospitalizations, and mortality [62,63]. Those with longer duration of bronchiectasis and proton pump inhibitor usage were shown to be associated with a higher risk of pseudomonas colonization [64]. Long-term use of antibiotics for those with chronic pseudomonas colonized patients has been studied for decades, especially via inhaled or nebulized route, as it may directly affect the airway, with limited systemic side effects [65-72]. The main goals of these studies were to decrease bacterial burden, enhance the health-related quality of life, and lower exacerbation and hospitalization risk. A meta-analysis performed by Laska et al. [73] reported that inhaled antibiotics is associated with the reduction of bacterial load (−2.32 log unit, p<0.0001), increased bacterial eradication (odds ratio [OR], 3.36; p=0.0010), re-duction of exacerbation frequency (RR, 0.81; p=0.020), and prolonged time to first exacerbation (HR, 0.83; p=0.028).
In the BTS guideline, nebulized colistin was primarily recommended in bronchiectasis patients who suffer from exacerbation three or more times per year and have pseudomonas colonization [18]. It was supported by a randomized controlled study by Haworth et al. [67] that showed delayed time to first exacerbation in adherent group with inhaled colistin, compared to control group (168 days vs. 103 days, p=0.038). Also, pseudomonas bacterial density and SGRQ score were improved in the inhaled colistin group. Inhaled gentamicin was advocated in the guideline as a second line alternative to colistin in frequent exacerbators [18]. The baseline study was a randomized controlled trial by Murray et al. [65] that showed the long-term use of nebulized gentamicin significantly lowered bacterial load and sputum purulence, and improved exercise capacity and health-related quality of life. Furthermore, the nebulized gentamicin group showed decreased exacerbation risk (p<0.0001) and delayed time to first exacerbation (p=0.02).
Other inhaled antibiotics, including ciprofloxacin, tobramycin, aztreonam, and ceftazidime, have been studied in chronic pseudomonas colonized bronchiectasis patients with frequent exacerbations [66,68-72,74-77]. Two randomized controlled studies (RESPIRE 1 and 2) were published to evaluate the benefit of ciprofloxacin dry powder for inhalation (DPI) in non-cystic fibrosis bronchiectasis patients with two or more exacerbation per year and having positive sputum culture for one or more pre-specified pathogens, including Pseudomonas aeruginosa, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, Streptococcus pneumoniae, Stenotrophomonas maltophilia, and Burkholderia cepacia [68,69]. Patients were randomly assigned as 2:1 to twice-daily ciprofloxacin DPI 32.5 mg or placebo group, and treatment regimens consisted of on/off treatment cycle of (14 and 28 days) for 48 weeks. In RESPIRE 1, treatment with ciprofloxacin DPI 14 days on/off regimen showed significant delay in time to first exacerbation, compared to the pooled placebo (median time >336 days vs. 186 days: HR, 0.53; p=0.0005), and decreased exacerbation frequency compared to the matching placebo (incidence rate ratio [IRR], 0.61; p=0.0061). However, ciprofloxacin DPI 28 days on/off regimen failed to show statistical significance in clinical outcomes, compared to placebo [69]. However, in RESPIRE 2, neither treatment regimen showed improvement in clinical outcomes, including time to first exacerbation and exacerbation frequency, although the treatment group tended to have a lower risk of exacerbations. The safety profile of ciprofloxacin DPI was acceptable in both studies [68].
The use of inhaled liposomal ciprofloxacin via nebulizer in bronchiectasis with two or more exacerbations and chronic pseudomonas colonization was evaluated in two phase III randomized controlled studies (ORBIT-3 and ORBIT-4) [70]. This was the advance study of the phase II trial (ORBIT-2 study) by Serisier et al. [66], which showed reduction of bacterial density and delayed time to first exacerbation with favorable safety profile in the treatment group, compared to placebo group. In ORBIT-3 and ORBIT-4, patients were 2:1 allocated to inhaled liposomal ciprofloxacin and placebo treatment, which were given 28 days on/off cycle for 48 weeks. In ORBIT-3 trial, time to first exacerbation (214 days vs. 136 days, p=0.97) and exacerbation frequency (RR, 0.73; p=0.26) between the two groups were not statistically significant. However, the ORBIT-4 trial showed significant differences in both time to first exacerbation (230 days vs. 158 days, p=0.032) and exacerbation frequency (RR, 0.63; p=0006). In pooled analysis, time to first exacerbation was not significant (222 days vs. 157 days, p=0.074), but exacerbation frequency was lower in the treatment group (RR, 9.73; p=0.0011). Sputum bacterial density was decreased in the 28 days “on” period, and increased in the “off” period. The following post hoc analysis showed that respiratory symptoms improved in the on-treatment periods, while the treatment effect diminished in the off treatment periods [74].
Although a meta-analysis clearly demonstrated the efficacy of inhaled antibiotics in bronchiectasis [73], individual randomized trials showed conflicting results [68-70,78]. There may be multiple explanations for this inconsistency [79]. First, baseline bacterial load may have impacted the results; those with high bacterial load may have more benefits from the treatment [80]. Second, drug administration schedule, whether it was cyclical or continuous, may have been the important aspect. Previous trials have reported more inconsistent results in using cyclical treatment, compared to continuous treatments [65,67,74,76]. Third, in using time to first exacerbation as the primary outcome, previous exacerbation history may have been a confounding factor, which is one of the most important predictors for future exacerbation [68-70,79]. Fourth, short duration of the drug administration may have shown negative results in some studies [78]. Finally, there may have been some patient factors, including recall bias, and cultural/geographical factors (e.g., tolerance of symptoms, prevalent comorbidities) [79].
Tobramycin is an antipseudomonal aminoglycoside; its inhalation has been studied for beneficial effect on non-cystic fibrosis bronchiectasis patients with chronic pseudomonas infection. A meta-analysis, which analyzed five studies with 211 participants included, showed transitory decrease of pseudomonas density in the inhaled tobramycin group, compared to control [72]. Furthermore, the inhaled tobramycin group lowered hospitalization rate, but failed to show reduction of exacerbation frequency. There were some limitations of small sample size and lack of proper control. To overcome these limitations, Guan et al. [81] recently published a double-blind randomized placebo-controlled phase III trial of tobramycin inhalation solution (TIS) in patients with bronchiectasis with chronic pseudomonas infection. A total of 339 patients (167 TIS group, and 172 placebo group) were enrolled, and two cycles of 28 days on and off treatments were administered via vibrating-mesh nebulizer. The TIS group showed significant reduction in bacterial density, 24 hours sputum volume, and purulence score, compared to control group. The Quality-of-Life Bronchiectasis Respiratory Symptoms score was improved in the TIS group (adjusted mean difference, 7.91; p<0.001), and more patients showed negative conversion of P. aeruginosa in sputum culture (29.3% vs. 10.6%). The safety profiles were comparable between the two groups. A dry powder formulation device, tobramycin inhalation powder (TIP) TOBI Podhaler (Novartis AG, Basel, Switzerland), was approved for cystic fibrosis patients with chronic P. aeruginosa colonization [76]. Recently, a phase II double-blind, randomized study were performed to evaluate the efficacy and safety of the TOBI Podhaler in patients with bronchiectasis patients with chronic pseudomonas infection. The TIP group, in both continuous and cyclical treatment, showed greater reduction of P. aeruginosa sputum density compared to placebo in a dose-dependent manner, without any safety issues. Other antibiotics, including aztreonam or ceftazidime, have been studied, and more evidence needs to be collected [71,77,78].
The current guidelines recommend the long-term use of macrolide in bronchiectasis patients with three or more exacerbations per year, primarily in those without pseudomonas colonization [18,19]. For those with pseudomonas colonization, inhaled antibiotics are recommended primarily, and macrolide alternatively. The beneficial effects of macrolides in bronchiectasis are not only associated with the reduction of bacterial burden, but also with immunomodulatory effects that alleviate neutrophilic inflammation and facilitate ciliary movement to enhance mucociliary clearance [82]. Chalmers et al. [82] recently published an individual participant data meta-analysis to evaluate long-term macrolide use in those with adult bronchiectasis. From three randomized controlled studies [83-85], the clinical data of 341 patients were analyzed. Macrolide use was associated with decreased exacerbation frequency (adjusted IRR, 0.49; p<0.0001), extended time to first exacerbation (adjusted HR, 0.46; p<0.0001), and improved SGRQ score (mean 2.93 points, p=0.048). A notable finding is that the reduction of exacerbation frequency was significant in all pre-specified subgroups, including those with P. aeruginosa infection (IRR, 0.36; p=0.0044). The beneficial effect on those with P. aeruginosa infection may be associated with the immunomodulatory effect of macrolide [82]. In addition, the effect of macrolides on bacterial virulence factor, such as quorum sensing, may be a key factor of the therapeutic effect [86]. Quorum sensing is a process by which bacteria communicate with each other, and coordinate their behavior through small signal molecules called autoinducers, which is important for the formation of bacterial biofilm. Macrolides have been shown to interfere with quorum sensing, thereby reducing the formation of biofilms, which may result in the prevention of exacerbations. Considering the relatively lower RR in the use of long-term macrolide compared to inhaled antibiotics in the aforementioned meta-analysis, the use of long-term macrolides in pseudomonas colonized bronchiectasis patients should be considered as the first line therapy [73,82]. Further head-to-head studies are needed.
Long-term use of macrolides is not only recommended in bronchiectasis patients, but in COPD patients with frequent exacerbation [87]. In GOLD 2023 guideline, azithromycin is indicated in former smoker with frequent exacerbations despite the use of triple therapy or dual bronchodilator (blood eosinophil <100 cells/μL). A single center study reported the effect of azithromycin on COPD patients according to the presence of bronchiectasis [88]. After the 12-month follow-up, those with bronchiectasis showed significantly lower incidence of ≥2 moderate or ≥1 severe exacerbation (46.5% vs. 87.5%, p=0.005), and more likely to be symptomatic responder (≥2 points decrement from the initial COPD Assessment Test [CAT] score) (68.2% vs. 16.7%, p=0.004), compared to those without bronchiectasis. Despite the relatively small number of patients enrolled in this study (n=59), the use of azithromycin in COPD can be considered in those who have concomitant bronchiectasis.
Although there are prominent benefits in the use of long-term macrolides in bronchiectasis patients, several adverse effects should be accounted for; gastrointestinal symptoms (diarrhea or abdominal discomfort), increased antibiotic resistance, hearing loss, and QT prolongation have been reported as possible side effects of the long-term use of the antibiotics [82]. Furthermore, recent studies reported the possible association of long-term macrolide therapy and increased incidence of non-tuberculous mycobacteria pulmonary disease (NTM-PD). In a 10-year national cohort study in Korea, the use of macrolide was associated with the increased risk of NTM-PD for OR of 6.82 (p<0.001) in multivariate analysis [89]. Regarding the potential harm of macrolides in NTM-PD, it may be important to obtain sputum acid-fast bacillus (AFB) test results before the commencement of long-term macrolide therapy to prevent macrolide-resistant NTM-PD, which is a difficult-to-treat infection [18]. However, in analysis of the United States Bronchiectasis and NTM Research Registry (BRR), the macrolide group showed lesser positivity of mycobacterial culture, compared to the non-macrolide group, which suggests the prophylactic or protective effect of macrolide against NTM-PD [90]. Further analysis of large and more well-designed studies is needed to conclude the beneficial or harmful effect of long-term macrolide therapy in the incidence of NTM-PD.
In 2017, the expert panel has established a consensus definition of bronchiectasis exacerbation for clinical research using the Delphi method [91]. The exacerbation was defined by an event with a deterioration in three or more of the following key symptoms for at least 48 hours: cough, sputum volume and/or consistency; sputum purulence, breathlessness and/or exercise tolerance, fatigue and/or malaise, and hemoptysis. Also, clinician decision of modification in the bronchiectasis treatment plan was necessary. However, while this definition was based on patients in Europe, North America, Australia and South Africa, there was no representation of the Asian population; further validation is needed.
For those with an exacerbation of bronchiectasis, courses of 14 to 21 days of systemic antibiotics are recommended in previous guidelines, although the evidence of this duration is poor [18,19]. Shorter duration of treatment (e.g., 7 days) may be sufficient in those with mild bronchiectasis, mild exacerbation, those related to antibiotics-sensitive pathogens, or those who rapidly recover to their baseline state [19]. Recently, there was a proof-of-concept randomized controlled trial investigating the feasibility of shorter duration of antibiotics therapy based on bacterial load [92]. Patients were randomized either to a group prescribed with 14 days of intravenous antibiotics, or to a bacterial load-guided group (BLGG). In BLGG, antibiotics were discontinued when the bacterial load was less than 106 colony-forming unit (CFU)/mL on either day 7 or day 10. There were no significant differences in clinical improvement between the two groups at day 21; however, exacerbation risk was paradoxically higher in the 14-day group, compared to the BLGG group (HR, 1.80; p=0.009). The current guideline also suggests that intravenous antibiotics should be considered for patients who are severely ill, who have resistant pathogens, or who have not responded to prior oral antibiotics [18].
ACTs, including manual technique, intermittent positive pressure breathing, and non-invasive ventilation, were also recommended in the BTS guideline [18]. However, further randomized controlled studies are required to investigate the benefit of ACT in the exacerbation of bronchiectasis.
Although this narrative review presented various treatments that have shown benefits in bronchiectasis patients, there are still unmet needs for treatments for bronchiectasis, and to identify the patient group that will be most responsive to each treatment. The current development of novel therapies is mainly focused on three components of the vicious cycle of bronchiectasis, which cycle consists of bacterial infection, neutrophilic inflammation, and impaired mucociliary clearance [61].
The inhaled antibiotics that have been mostly used are off-label, or currently under investigation [93]. Overall, inhaled antibiotics have shown benefits, especially in sputum volume, bacterial load, quality of life, and exacerbation rates; however, inconsistent results have also been reported between each randomized controlled study [79]. Further research should focus on optimizing antimicrobial therapy in bronchiectasis patients. Following the promising outcomes observed with brensocatib as a potential treatment for bronchiectasis, other drugs targeting neutrophiliic inflammation are under investigation (NCT03218917, NCT04322929), while the result of phase 3 trial of brensocatib is also upcoming (NCT04594369). To enhance mucociliary clearance, ACT and mucoactive drugs have been used in the clinical field; however, they were supported by little data. Large randomized open label trial (CLEAR) is ongoing in the UK for hypertonic saline and carbocysteine over 52 weeks in bronchiectasis (NCT04140214). Cystic fibrosis transmembrane conductance regulator (CFTR) modifier may play some role in non-cystic fibrosis bronchiectasis, which has shown remarkable outcome in cystic fibrosis. Theoretically, CFTR modifier could be efficacious if CFTR dysfunction were present at the epithelial level without genetic mutation in bronchiectasis [61]. Bronchiectasis is a heterogenetic disease, and future research should move toward endophenotypic assessment and personalized medicine.
Bronchiectasis is a common chronic pulmonary disease that entails substantial respiratory symptoms and frequent exacerbations. Bronchiectasis has not received much attention, but clinical outcomes and treatments of bronchiectasis have been recently widely studied. Among non-pharmacological treatments, physiotherapy, including ACT, is one of the most important therapies. Well-performed ACT is associated with improved sputum clearance, enhanced quality of life, and decreased mortality. Increasing evidence of the role of PR in bronchiectasis has shown that it may improve exercise tolerance and lung function. The role of surgery is limited in bronchiectasis. Those with severe localized lesion who are not responsive to all medical treatments, or those with severe complications, may be potential candidates for surgery. However, there may be complication resulting from surgery, and careful patient selection is needed.
Many studies have sought to identify the optimal pharmacological treatment for bronchiectasis patients. Mucoactive drugs have shown potential to reduce exacerbation risk in bronchiectasis patients with frequent exacerbations; however, further well-designed studies are needed. Anti-inflammatory drug targeting neutrophilic inflammation (i.e., brensocatib) has shown promising outcomes, especially in reducing exacerbation risk, and further phase 3 trials are ongoing. For eosinophilic bronchiectasis, the use of ICS was shown to be effective in reducing exacerbation; however, further larger studies are needed. Bronchodilators are presented to increase lung functions in bronchiectasis patients in BCOS patients. Inhaled antibiotics and macrolides are recommended in bronchiectasis patients with three or more exacerbations per the previous year, the former in those with chronic pseudomonas infection, and the latter without. However, recent evidence suggests that macrolide may have more protective effect against exacerbation than inhaled antibiotics in those with chronic pseudomonas infection; yet further studies are needed to conclude the ideal and patient-tailored therapy for bronchiectasis patients.
Fig. 1.
Management of bronchiectasis. ACT: airway clearance technique; ACBT: the active cycle of breathing technique; PD: pulmonary disease; OPEP: oscillating positive expiratory pressure; QOL: quality of life; NAC: N-acetylcysteine; FEV1: forced expiratory volume in 1 second; BCOS: bronchiectasis and COPD overlap syndrome; ICS: inhaled corticosteroid; PDE4: phosphodiesterase 4.
REFERENCES
1. Macfarlane L, Kumar K, Scoones T, Jones A, Loebinger MR, Lord R. Diagnosis and management of non-cystic fibrosis bronchiectasis. Clin Med (Lond) 2021;21:e571-7.



2. Pasteur MC, Bilton D, Hill AT; British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58.


3. Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J 1997;10:2784-7.


5. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis 2017;14:377-84.




6. Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016;47:186-93.



7. Monteagudo M, Rodriguez-Blanco T, Barrecheguren M, Simonet P, Miravitlles M. Prevalence and incidence of bronchiectasis in Catalonia, Spain: a population-based study. Respir Med 2016;121:26-31.


8. Choi H, Lee H, Ra SW, Jang JG, Lee JH, Jhun BW, et al. Developing a diagnostic bundle for bronchiectasis in South Korea: a modified Delphi consensus study. Tuberc Respir Dis (Seoul) 2022;85:56-66.




9. Choi H, Yang B, Nam H, Kyoung DS, Sim YS, Park HY, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J 2019;54:1900194.


10. Diel R, Chalmers JD, Rabe KF, Nienhaus A, Loddenkemper R, Ringshausen FC. Economic burden of bronchiectasis in Germany. Eur Respir J 2019;53:1802033.


11. Choi H, Yang B, Kim YJ, Sin S, Jo YS, Kim Y, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep 2021;11:7126.




12. Kim Y, Kim K, Rhee CK, Ra SW. Increased hospitalizations and economic burden in COPD with bronchiectasis: a nationwide representative study. Sci Rep 2022;12:3829.




13. Yang B, Jang HJ, Chung SJ, Yoo SJ, Kim T, Kim SH, et al. Factors associated with bronchiectasis in Korea: a national database study. Ann Transl Med 2020;8:1350.



14. Sin S, Yun SY, Kim JM, Park CM, Cho J, Choi SM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res 2019;20:271.




15. Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index: an international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85.



16. Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med 2018;197:1410-20.


17. Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Inflammatory responses, spirometry, and quality of life in subjects with bronchiectasis exacerbations. Respir Care 2015;60:1180-9.


18. Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn SJ, Floto AR, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax 2019;74(Suppl 1):1-69.

19. Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629.


20. Murray MP, Pentland JL, Hill AT. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:1086-92.


21. O’Neill B, Bradley JM, McArdle N, MacMahon J. The current physiotherapy management of patients with bronchiectasis: a UK survey. Int J Clin Pract 2002;56:34-5.


22. Thompson CS, Harrison S, Ashley J, Day K, Smith DL. Randomised crossover study of the Flutter device and the active cycle of breathing technique in non-cystic fibrosis bronchiectasis. Thorax 2002;57:446-8.



23. Patterson JE, Bradley JM, Hewitt O, Bradbury I, Elborn JS. Airway clearance in bronchiectasis: a randomized cross-over trial of active cycle of breathing techniques versus acapella. Respiration 2005;72:239-42.



24. Eaton T, Young P, Zeng I, Kolbe J. A randomized evaluation of the acute efficacy, acceptability and tolerability of flutter and active cycle of breathing with and without postural drainage in non-cystic fibrosis bronchiectasis. Chron Respir Dis 2007;4:23-30.



25. Lee AL, Williamson HC, Lorensini S, Spencer LM. The effects of oscillating positive expiratory pressure therapy in adults with stable non-cystic fibrosis bronchiectasis: a systematic review. Chron Respir Dis 2015;12:36-46.



26. Munoz G, de Gracia J, Buxo M, Alvarez A, Vendrell M. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. Eur Respir J 2018;51:1701926.


27. Huang HY, Chung FT, Lin CY, Lo CY, Huang YT, Huang YC, et al. Influence of comorbidities and airway clearance on mortality and outcomes of patients with severe bronchiectasis exacerbations in Taiwan. Front Med (Lausanne) 2022;8:812775.



28. Liu Y, Lu HW, Gu SY, Wang WW, Ge J, Jie ZJ, et al. Bronchoscopic airway clearance therapy for acute exacerbations of bronchiectasis. EBioMedicine 2021;72:103587.



29. Ora J, Prendi E, Ritondo BL, Pata X, Spada F, Rogliani P. Pulmonary rehabilitation in noncystic fibrosis bronchiectasis. Respiration 2022;101:97-105.



30. Yang F, Gao L, Wang Q, Deng W, Gao W. Effect of exercise-based pulmonary rehabilitation in patients with bronchiectasis: a meta-analysis. Respir Med Res 2022;81:100910.


31. Cedeno de Jesus S, Almadana Pacheco V, Valido Morales A, Muniz Rodriguez AM, Ayerbe Garcia R, Arnedillo-Munoz A. Exercise capacity and physical activity in non-cystic fibrosis bronchiectasis after a pulmonary rehabilitation home-based programme: a randomised controlled trial. Int J Environ Res Public Health 2022;19:11039.



32. Candemir I, Ergun P, Satar S, Karamanli H, Kaymaz D, Demir N. Efficacy of pulmonary rehabilitation for bronchiectasis and related factors: which patients should receive the most treatment? Adv Respir Med 2021;89:15-22.


33. Chalmers JD, Crichton ML, Brady G, Finch S, Lonergan M, Fardon TC. Pulmonary rehabilitation after exacerbation of bronchiectasis: a pilot randomized controlled trial. BMC Pulm Med 2019;19:85.




34. Alcaraz-Serrano V, Gimeno-Santos E, Scioscia G, Gabarrus A, Navarro A, Herrero-Cortina B, et al. Association between physical activity and risk of hospitalisation in bronchiectasis. Eur Respir J 2020;55:1902138.


35. Xu JF, Gao YH, Song YL, Qu JM, Guan WJ. Research advances and clinical management of bronchiectasis: Chinese perspective. ERJ Open Res 2022;8:00017-2022.



36. Selman A, Merhej H, Nakagiri T, Zinne N, Goecke T, Haverich A, et al. Surgical treatment of non-cystic fibrosis bronchiectasis in Central Europe. J Thorac Dis 2021;13:5843-50.



37. O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998;113:1329-34.


38. Bilton D, Daviskas E, Anderson SD, Kolbe J, King G, Stirling RG, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest 2013;144:215-25.


39. Bilton D, Tino G, Barker AF, Chambers DC, De Soyza A, Dupont LJ, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax 2014;69:1073-9.


40. Gao YH, Abo Leyah H, Finch S, Lonergan M, Aliberti S, De Soyza A, et al. Relationship between symptoms, exacerbations, and treatment response in bronchiectasis. Am J Respir Crit Care Med 2020;201:1499-507.


41. Maiz Carro L, Martinez-Garcia MA. Nebulized hypertonic saline in noncystic fibrosis bronchiectasis: a comprehensive review. Ther Adv Respir Dis 2019;13:1753466619866102.


42. Qi Q, Ailiyaer Y, Liu R, Zhang Y, Li C, Liu M, et al. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): a randomized controlled trial. Respir Res 2019;20:73.




43. Luo A, Liu X, Hu Q, Yang M, Jiang H, Liu W. Efficacy of N-acetylcysteine on idiopathic or postinfective non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis protocol. BMJ Open 2022;12:e053625.



44. Liao Y, Wu Y, Zi K, Shen Y, Wang T, Qin J, et al. The effect of N-acetylcysteine in patients with non-cystic fibrosis bronchiectasis (NINCFB): study protocol for a multicentre, double-blind, randomised, placebo-controlled trial. BMC Pulm Med 2022;22:401.




45. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018;392:880-90.



46. Dente FL, Bilotta M, Bartoli ML, Bacci E, Cianchetti S, Latorre M, et al. Neutrophilic bronchial inflammation correlates with clinical and functional findings in patients with noncystic fibrosis bronchiectasis. Mediators Inflamm 2015;2015:642503.




47. Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2017;195:1384-93.



48. Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020;383:2127-37.


49. Hakansson KE, Fjaellegaard K, Browatzki A, Donmez Sin M, Ulrik CS. Inhaled corticosteroid therapy in bronchiectasis is associated with all-cause mortality: a prospective cohort study. Int J Chron Obstruct Pulmon Dis 2021;16:2119-27.


50. Shoemark A, Shteinberg M, De Soyza A, Haworth CS, Richardson H, Gao Y, et al. Characterization of eosinophilic bronchiectasis: a European multicohort study. Am J Respir Crit Care Med 2022;205:894-902.


51. Aliberti S, Sotgiu G, Blasi F, Saderi L, Posadas T, Martinez Garcia MA. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J 2020;56:2000453.


52. Martinez-Garcia MA, Posadas T, Sotgiu G, Blasi F, Saderi L, Aliberti S. Role of inhaled corticosteroids in reducing exacerbations in bronchiectasis patients with blood eosinophilia pooled post-hoc analysis of 2 randomized clinical trials. Respir Med 2020;172:106127.


53. Rademacher J, Konwert S, Fuge J, Dettmer S, Welte T, Ringshausen FC. Anti-IL5 and anti-IL5Rα therapy for clinically significant bronchiectasis with eosinophilic endotype: a case series. Eur Respir J 2020;55:1901333.


54. Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66.


55. Juthong S, Panyarath P. Efficacy of roflumilast in bronchiectasis patients with frequent exacerbations: a double-blinded, randomized, placebo-controlled pilot clinical trial. Tuberc Respir Dis (Seoul) 2022;85:67-73.




56. Sobala R, De Soyza A. Bronchiectasis and chronic obstructive pulmonary disease overlap syndrome. Clin Chest Med 2022;43:61-70.


57. Kim SH, Kim C, Jeong I, Lee SJ, Kim TH, Lee CY, et al. Chronic obstructive pulmonary disease is associated with decreased quality of life in bronchiectasis patients: findings from the KMBARC Registry. Front Med (Lausanne) 2021;8:722124.



58. Jeong HJ, Lee H, Carriere KC, Kim JH, Han JH, Shin B, et al. Effects of long-term bronchodilators in bronchiectasis patients with airflow limitation based on bronchodilator response at baseline. Int J Chron Obstruct Pulmon Dis 2016;11:2757-64.




59. Lee SY, Lee JS, Lee SW, Oh YM. Effects of treatment with long-acting muscarinic antagonists (LAMA) and long-acting beta-agonists (LABA) on lung function improvement in patients with bronchiectasis: an observational study. J Thorac Dis 2021;13:169-77.



60. Jayaram L, Vandal AC, Chang CL, Lewis C, Tong C, Tuffery C, et al. Tiotropium treatment for bronchiectasis: a randomised, placebo-controlled, crossover trial. Eur Respir J 2022;59:2102184.



61. Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018;6:715-26.


62. Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015;12:1602-11.

63. Martinez-Garcia MA, Oscullo G, Posadas T, Zaldivar E, Villa C, Dobarganes Y, et al. Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin Microbiol Infect 2021;27:428-34.


64. Kwok WC, Ho JC, Tam TC, Ip MS, Lam DC. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir Res 2021;22:132.




65. Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011;183:491-9.


66. Serisier DJ, Bilton D, De Soyza A, Thompson PJ, Kolbe J, Greville HW, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax 2013;68:812-7.



67. Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014;189:975-82.



68. Aksamit T, De Soyza A, Bandel TJ, Criollo M, Elborn JS, Operschall E, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018;51:1702053.


69. De Soyza A, Aksamit T, Bandel TJ, Criollo M, Elborn JS, Operschall E, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018;51:1702052.


70. Haworth CS, Bilton D, Chalmers JD, Davis AM, Froehlich J, Gonda I, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med 2019;7:213-26.


71. Crichton ML, Lonergan M, Barker AF, Sibila O, Goeminne P, Shoemark A, et al. Inhaled aztreonam improves symptoms of cough and sputum production in patients with bronchiectasis: a post hoc analysis of the AIR-BX studies. Eur Respir J 2020;56:2000608.


72. Sangiovanni S, Morales EI, Fernandez-Trujillo L. Inhaled tobramycin for chronic infection with pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. Respir Med 2021;176:106283.


73. Laska IF, Crichton ML, Shoemark A, Chalmers JD. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Lancet Respir Med 2019;7:855-69.


74. Chalmers JD, Cipolla D, Thompson B, Davis AM, O’Donnell A, Tino G, et al. Changes in respiratory symptoms during 48-week treatment with ARD-3150 (inhaled liposomal ciprofloxacin) in bronchiectasis: results from the ORBIT-3 and -4 studies. Eur Respir J 2020;56:2000110.


75. Elborn JS, Blasi F, Haworth CS, Ballmann M, Tiddens HA, Murris-Espin M, et al. Bronchiectasis and inhaled tobramycin: a literature review. Respir Med 2022;192:106728.


76. Loebinger MR, Polverino E, Chalmers JD, Tiddens HA, Goossens H, Tunney M, et al. Efficacy and safety of TOBI Podhaler in Pseudomonas aeruginosa-infected bronchiectasis patients: iBEST study. Eur Respir J 2021;57:2001451.


77. Riveiro V, Casal A, Alvarez-Dobano JM, Lourido T, Suarez-Artime P, Rodriguez-Garcia C, et al. Response to inhaled ceftazidime in patients with non-cystic fibrosis bronchiectasis and chronic bronchial infection unrelated to Pseudomonas aeruginosa. Clin Respir J 2022;16:768-73.




78. Barker AF, O’Donnell AE, Flume P, Thompson PJ, Ruzi JD, de Gracia J, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014;2:738-49.


80. Sibila O, Laserna E, Shoemark A, Keir HR, Finch S, Rodrigo-Troyano A, et al. Airway bacterial load and inhaled antibiotic response in bronchiectasis. Am J Respir Crit Care Med 2019;200:33-41.


81. Guan WJ, Xu JF, Luo H, Xu XX, Song YL, Ma WL, et al. A double-blind randomized placebo-controlled phase 3 trial of tobramycin inhalation solution in adults with bronchiectasis with Pseudomonas aeruginosa infection. Chest 2023;163:64-76.


82. Chalmers JD, Boersma W, Lonergan M, Jayaram L, Crichton ML, Karalus N, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019;7:845-54.


83. Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251-9.


84. Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260-7.


85. Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660-7.


86. Burr LD, Rogers GB, Chen AC, Hamilton BR, Pool GF, Taylor SL, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non-cystic fibrosis bronchiectasis. an analysis from the bronchiectasis and low-dose erythromycin study trial. Ann Am Thorac Soc 2016;13:1697-703.

87. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report) [Internet]. Deer Park: GOLD; 2021 [cited 2023 May 26]. Available from: https://goldcopd.org/wpcontent/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
88. Choi Y, Shin SH, Lee H, Cho HK, Im Y, Kang N, et al. Favorable response to long-term azithromycin therapy in bronchiectasis patients with chronic airflow obstruction compared to chronic obstructive pulmonary disease patients without bronchiectasis. Int J Chron Obstruct Pulmon Dis 2021;16:855-63.




89. Yang B, Ryu J, Kim T, Jo YS, Kim Y, Park HY, et al. Impact of bronchiectasis on incident nontuberculous mycobacterial pulmonary disease: a 10-year national cohort study. Chest 2021;159:1807-11.


90. Metersky ML, Choate R; Bronchiectasis and NTM Research Registry Investigators. The association of long-term macrolide therapy and nontuberculous mycobacterial culture positivity in patients with bronchiectasis. Chest 2021;160:466-9.


91. Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017;49:1700051.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




