Diagnostic Accuracy of the Quidel Sofia Rapid Influenza Fluorescent Immunoassay in Patients with Influenza-like Illness: A Systematic Review and Meta-analysis
Article information
Abstract
Background
Although the Quidel Sofia rapid influenza fluorescent immunoassay (FIA) is widely used to identify influenza A and B, the diagnostic accuracy of this test remains unclear. Thus, the objective of this study was to determine the diagnostic performance of this test compared to reverse transcriptase-polymerase chain reaction.
Methods
A systematic literature search was performed using MEDLINE, EMBASE, and the Cochrane Central Register. Pooled sensitivity, specificity, diagnostic odds ratio (DOR), and a hierarchical summary receiver-operating characteristic curve (HSROC) of this test for identifying influenza A and B were determined using meta-analysis. A sensitivity subgroup analysis was performed to identify potential sources of heterogeneity within selected studies.
Results
We identified 17 studies involving 8,334 patients. Pooled sensitivity, specificity, and DOR of the Quidel Sofia rapid influenza FIA for identifying influenza A were 0.78 (95% confidence interval [CI], 0.71–0.83), 0.99 (95% CI, 0.98–0.99), and 251.26 (95% CI, 139.39–452.89), respectively. Pooled sensitivity, specificity, and DOR of this test for identifying influenza B were 0.72 (95% CI, 0.60–0.82), 0.98 (95% CI, 0.96–0.99), and 140.20 (95% CI, 55.92–351.54), respectively. The area under the HSROC for this test for identifying influenza A was similar to that for identifying influenza B. Age was considered a probable source of heterogeneity.
Conclusion
Pooled sensitivities of the Quidel Sofia rapid influenza FIA for identifying influenza A and B did not quite meet the target level (≥80%). Thus, caution is needed when interpreting data of this study due to substantial between-study heterogeneity.
Introduction
Influenza, an acute respiratory viral infection caused by influenza A or B virus, occurs mainly in the winter months throughout the world. It causes significant morbidity and mortality worldwide1,2. Adequate antiviral therapy can shorten the time of illness and reduce the duration of hospitalization and the risk of complications from influenza infections3. Clinical benefit of antiviral therapy is the greatest when it is started soon after the onset of influenza illness4. Therefore, rapid and accurate diagnosis of influenza infection is necessary in clinical practice.
Although polymerase chain reaction (PCR) has been used as the reference standard for diagnosing viral infections, performing PCR is relatively expensive. In addition, it requires technical expertise5. Alternatively, point-of-care rapid influenza diagnostic tests (RIDTs) can detect viral antigens by immunoassay and provide quick results within 30 minutes. They can facilitate antiviral therapy, reduce additional diagnostic tests and hospitalization therapy, and induce appropriate infection control measures6,7. A recent systematic review and meta-analysis for 162 diagnostic accuracy studies of RIDTs has revealed that traditional RIDTs have specificities higher than 98% with poor sensitivities (54.4% for influenza A and 53.2% for influenza B)5. Two classes of RIDTs, automated immunochromatographic antigen detection tests (digital immunoassays [DIAs]) and rapid nucleic acid amplification tests (NAATs), have been used since 20115. Pooled sensitivities for DIAs (80.0% for influenza A and 76.8% for influenza B) and rapid NAATs (91.6% for influenza A and 95.4% for influenza B) are significantly higher than those for traditional RIDTs5.
As a type of DIA, the Quidel Sofia rapid influenza fluorescent immunoassay (FIA) (Quidel Corp., San Diego, CA, USA) is a point-of-care test to detect influenza A and B in less than 15 minutes using a compact instrument (Sofia analyzer). Although the performance characteristics of this test to detect seasonal influenza virus strains have been established, the diagnostic accuracy of the Quidel Sofia rapid influenza FIA is not yet fully known. Thus, the objective of this systematic review and meta-analysis of clinical trial data was to investigate diagnostic properties of the Quidel Sofia rapid influenza FIA in patients with influenza like illness.
Materials and Methods
1. Data sources and search strategy
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses of Diagnostic Test Accuracy Studies statement8. A comprehensive search of three electronic databases (MEDLINE, EMBASE, and the Cochrane Central Register) up to July of 2020 was performed. Search terms for influenza included “Influenza, Human” [MeSh] OR “Influenza A virus” OR “Influenza B virus” OR “influenza” OR “flu”. Search terms for the tests included “rapid test*” OR “rapid diagnos*” OR “point-of-care test*” OR “immunoassay*” OR “immunochromatographic test*” OR “influenza FIA” OR “Quidel Sofia Influenza” OR “Rapid Detection Flu”. As this study was a systematic review of published articles, neither informed consent nor ethics approval was required. A manual search of references listed in relevant review articles was also conducted.
2. Study selection
We included studies that met the following inclusion criteria: (1) full-length reports published in peer-reviewed English language journals; (2) studies that evaluated the performance of the Quidel Sofia rapid influenza FIA, compared to a reference standard; (3) studies that included patients with influenza-like illness; and (4) studies that provided sufficient data to calculate absolute numbers of true-positive, false-positive, false-negative, and true-negative results. Review articles, case reports, commentaries, and studies reporting outcomes without raw data or peer review were excluded. Demographics and underlying diseases of participants were not restricted.
Influenza-like illness was defined as fever ≥38°C and any signs/symptoms of respiratory tract infection (e.g., cough, sputum, sore throat, wheezing, etc.). We allowed the followings as specimens: nasopharyngeal aspirates, swabs, or washes; nasal aspirates, swabs, or washes; saliva; and throat swabs. A reference standard was either a commercial reverse transcriptase–polymerase chain reaction (RT-PCR) or a laboratory-developed RT-PCR.
3. Data extraction and quality assessment
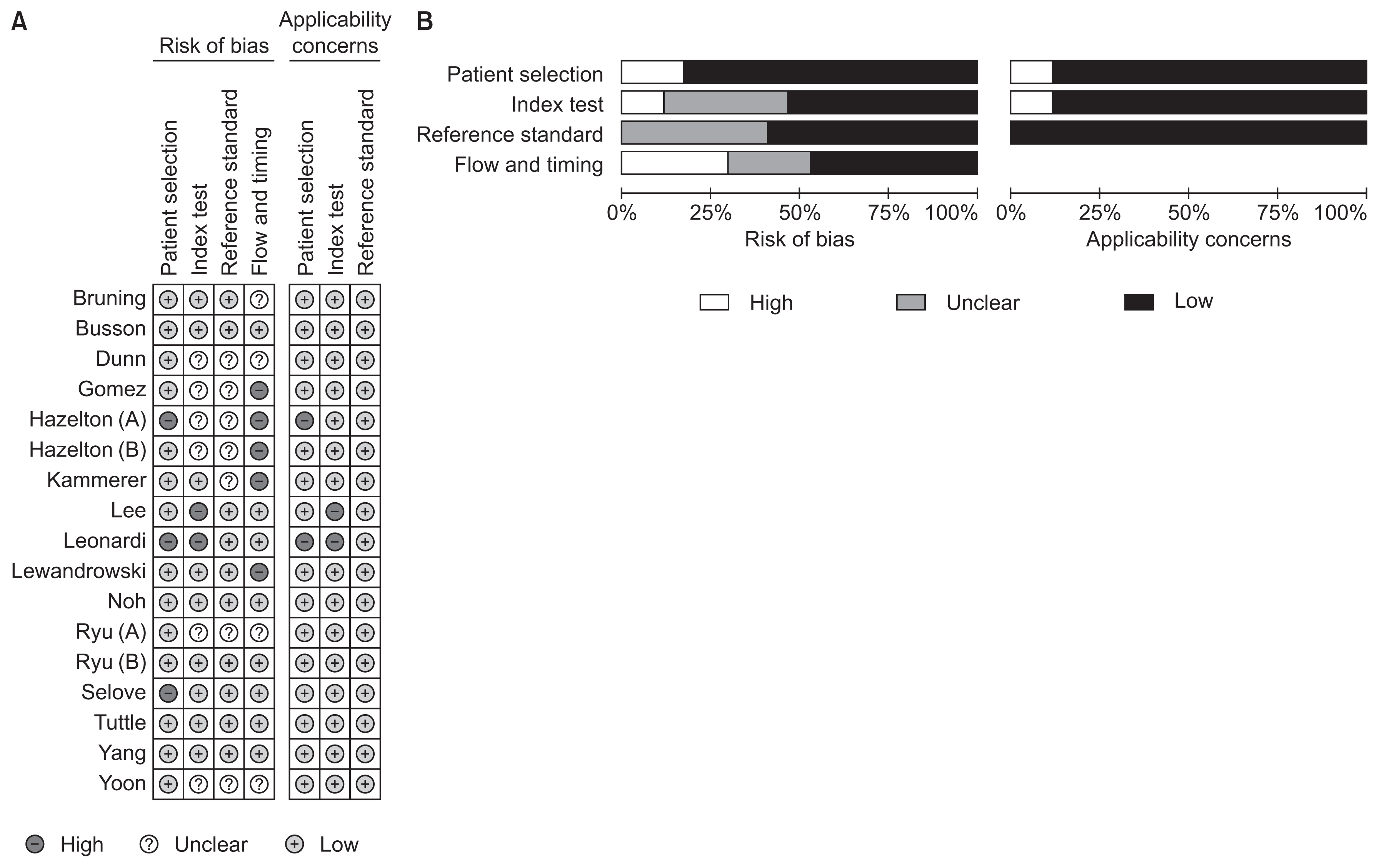
Two authors independently reviewed potentially relevant studies and each study according to predefined eligibility criteria, after which data were extracted. Any disagreements that arose during the process of study selection or data extraction were resolved by discussion. A predefined form was used to extract data from each study. The following data from each study included in the meta-analysis were extracted: author, year of publication, study design, place of study, number of participants, age, proportion of children, sex, study period, type of reference standard, type of specimens, and type of population. As recommended by the Cochrane Collaboration, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool was used to assess the risk of bias in diagnostic test accuracy9. Discrepancies were resolved by consensus between the two authors.
4. Data synthesis and statistical analysis
For diagnostic meta-analysis, random effects meta-analyses were performed to generate pooled estimates with 95% confidence intervals (CIs). Numbers of patients were extracted with true-positive, false-positive, false-negative, and true negative test results either directly or indirectly through a recalculation based on reported measures of accuracy in combination with the prevalence and sample size of the included study. Pooled sensitivity and specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the receiver-operating characteristic curve (AUC) were calculated as pooled estimates with a 95% CI10. Hierarchical summary receiver-operating characteristic curves (HSROCs) were also constructed. The heterogeneity was evaluated using the Higgins I2 statistics on a scale of 0–1. An I2>0.5 indicated a substantial level of between-study heterogeneity. To assess effects of potential sources of heterogeneity, subgroup analyses were performed using the following covariates to the model: study design (single vs. multicenter), number of participants (≥250 vs. <250), study period (influenza vs. non-specific season), and study population (children vs. adult). Pooled sensitivity and specificity estimates were calculated for each covariate. To investigate the effect of study quality, sensitivity analyses were performed. A p-value <0.05 was considered statistically significant. All statistical analyses were performed with Stata statistical software version 14.2 (Stata Corp LP, College Station, TX, USA) and Review Manager version 5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
1. Study search, characteristics of included studies, and quality of included studies
The literature search process is shown in Figure 1. Initially, 432 articles from PubMed, 1,310 articles from EMBASE, 356 articles from the Cochrane library, and an additional article from hand-searching were identified. After removing duplicate articles, 1,664 potentially eligible articles were screened. After reviewing titles and abstracts, 1,629 search records were removed and the remaining 35 articles were eligible for full text reading. Fifteen articles were excluded for reasons shown in Figure 1. With quantitative synthesis, seventeen studies were included in our final analysis11–27.
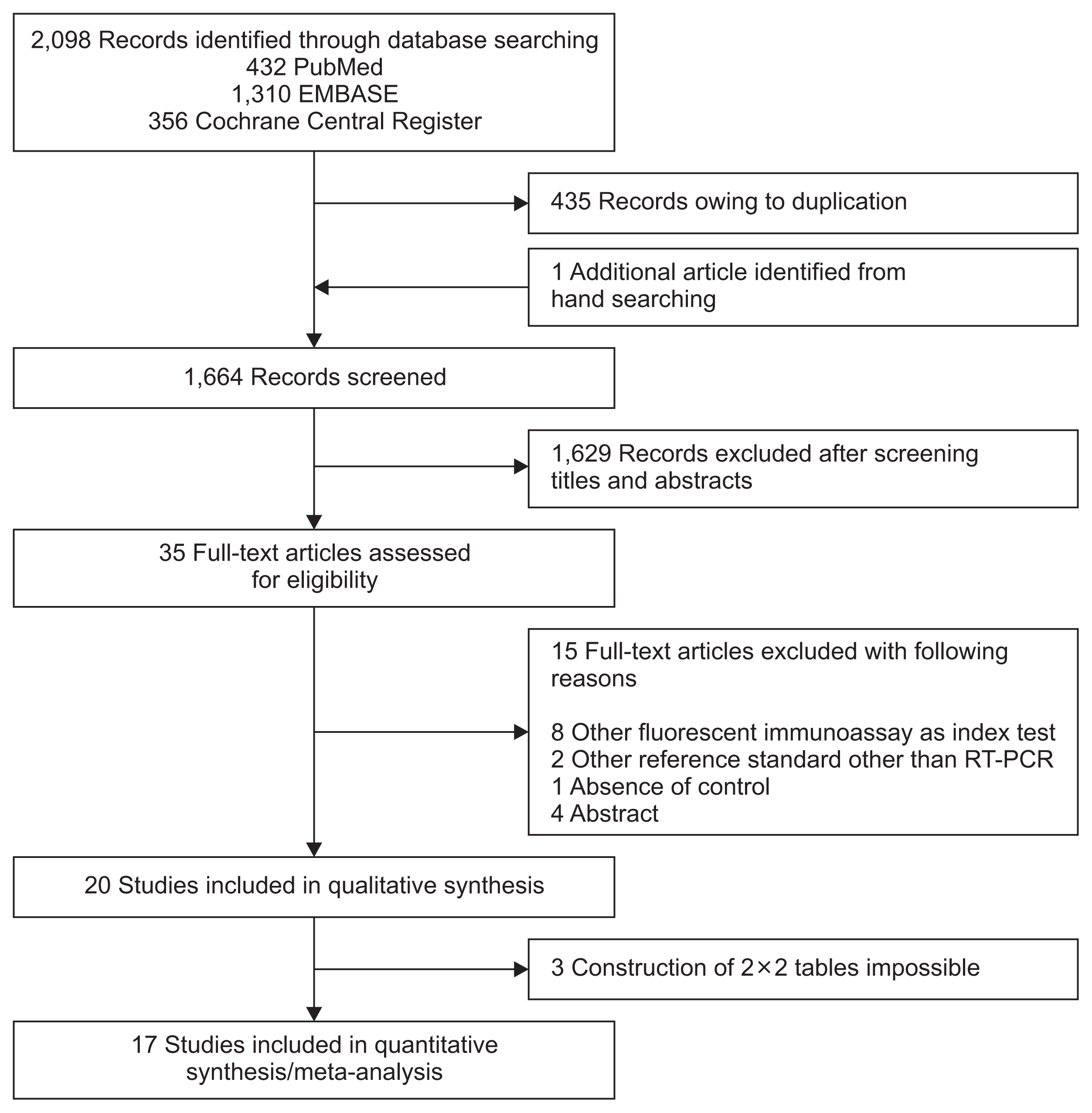
Flow diagram showing the identification of eligible studies. RT-PCR: reverse transcription polymerase chain reaction.
Features of included studies are summarized in Table 1. For influenza A, we identified 17 studies involving 8,334 participants. For influenza B, sixteen studies involving 7,909 subjects met the defined inclusion criteria. One study assessed influenza A infection only21. The number of patients in each trial ranged from 68 to 1,649. All studies were published between 2012 and 2018. Most studies evaluated combined populations of adults and children.
Results of the QUADAS-2 assessment are shown in Figure 2. For patient selection, the index test, and the reference standard domain, more than 50% of included studies were judged to have a low risk of bias. However, for the study flow and the timing domain, the risk of bias was high or unclear for 52.9% of the included studies because of unclear intervals between the index test and the reference standard. Considering our inclusion criteria, we had little concern for the applicability of results from selected studies for each domain.
2. Diagnostic accuracy of the Quidel Sofia rapid influenza FIA to identify influenza A and B
Figures 3 and 4 show paired forest plots of sensitivity and specificity of the Quidel Sofia rapid influenza FIA for detecting influenza A and B. The pooled sensitivity across studies of the Quidel Sofia rapid influenza FIA for identifying influenza A was 0.78 (95% CI, 0.71–0.83), with a pooled specificity of 0.99 (95% CI, 0.98–0.99). The pooled PLR and NLR were 56.99 (95% CI, 31.87–101.90) and 0.23 (95% CI, 0.18–0.29), respectively. The DOR for influenza A was 251.26 (95% CI, 139.39–452.89).
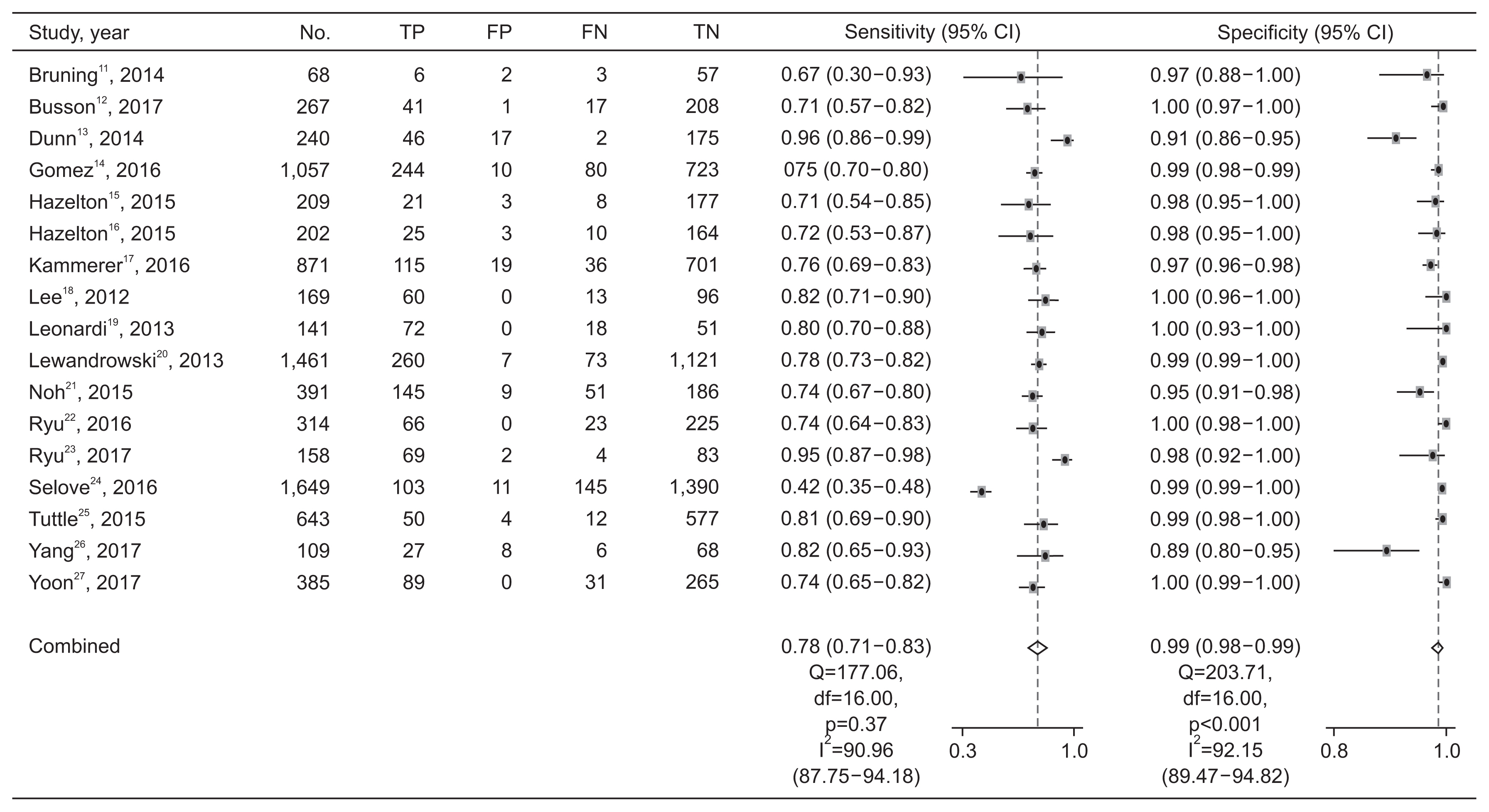
Paired forest plots of sensitivity and specificity of the Quidel Sofia rapid influenza fluorescent immunoassay for detecting influenza A. TP: true positive; FP: false positive; FN: false negative; TN: true negative; CI: confidence interval.
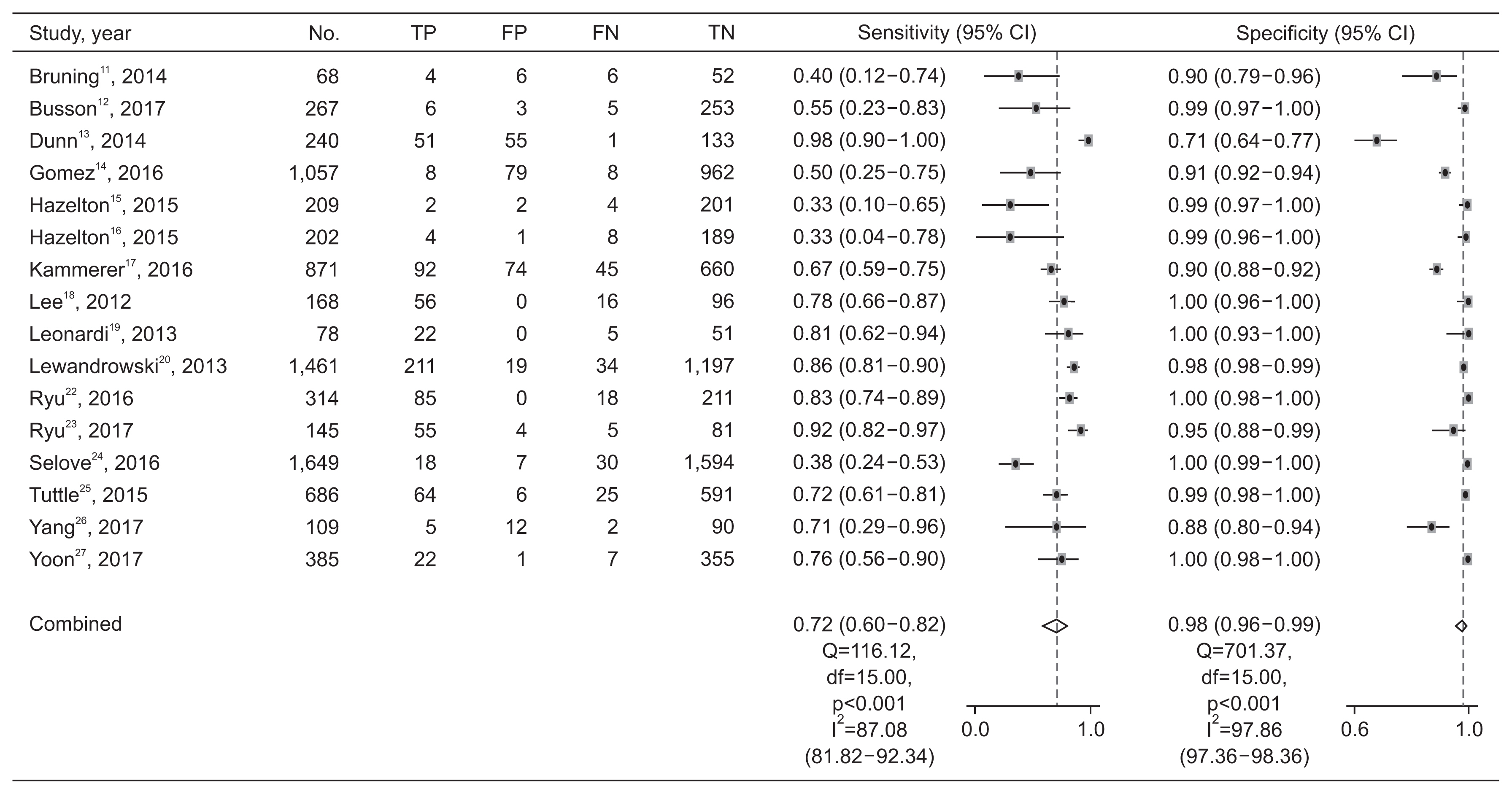
Paired forest plots of sensitivity and specificity of the Quidel Sofia rapid influenza fluorescent immunoassay for detecting influenza B. TP: true positive; FP: false positive; FN: false negative; TN: true negative; CI: confidence interval.
The pooled sensitivity across studies for identifying influenza B was 0.72 (95% CI, 0.60–0.82), with a pooled specificity of 0.98 (95% CI, 0.96–0.99). The pooled PLR and NLR were 40.08 (95% CI, 17.26–93.07) and 0.29 (95% CI, 0.19–0.42), respectively. The DOR for influenza B was 140.20 (95% CI, 55.92–351.54). The pooled sensitivity and specificity of the Quidel Sofia rapid influenza FIA for identifying influenza A and B were not significantly different (p=0.341 for sensitivity and p=0.206 for specificity). Figure 5 shows HSROCs for the index test. AUCs of the Quidel Sofia rapid influenza FIA for identifying influenza A and influenza B were similar (0.96 with 95% CI of 0.94–0.98 for influenza A vs. 0.95 with 95% CI of 0.92–0.96 for influenza B, p=0.166).
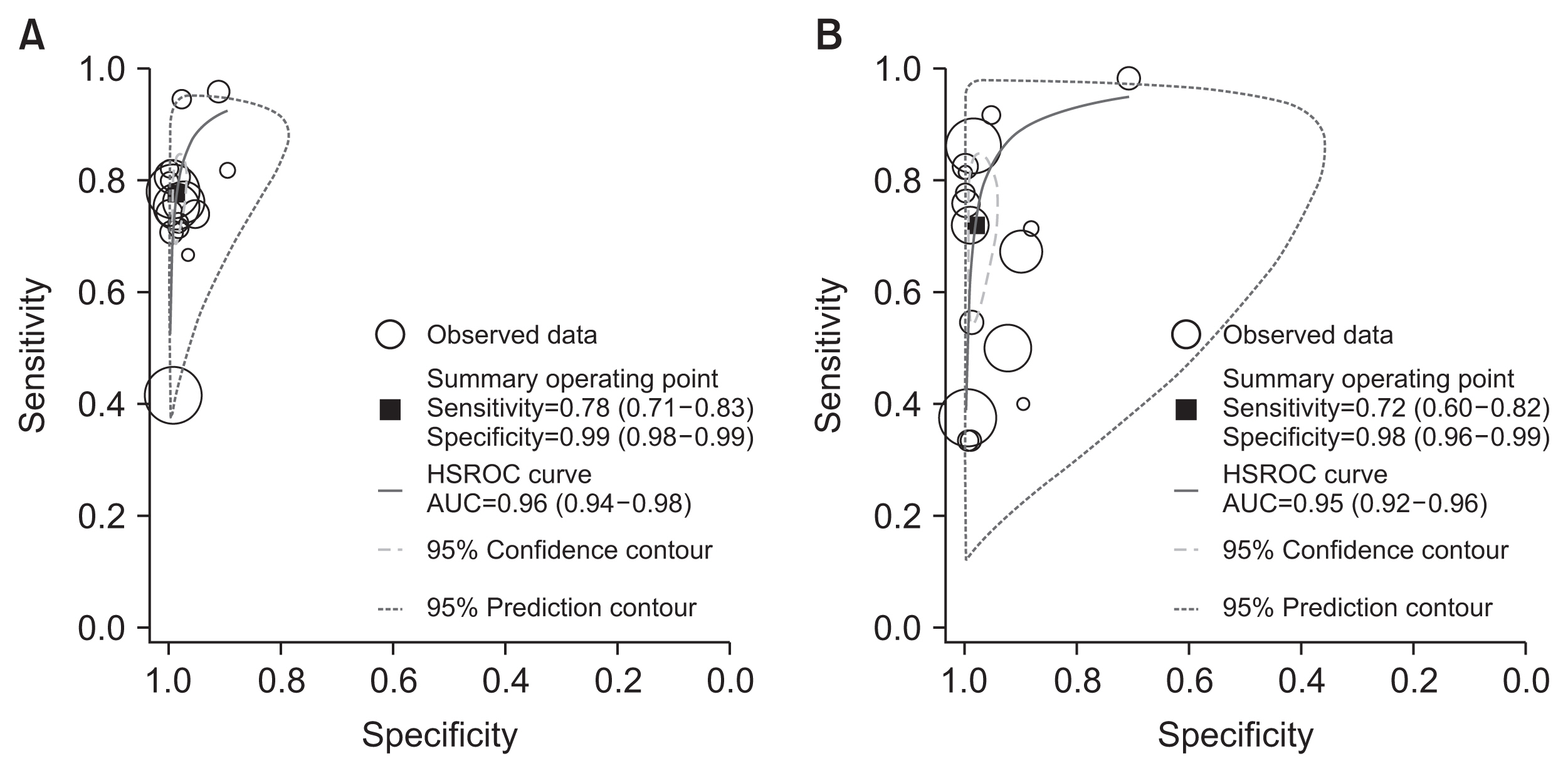
Hierarchical summary receiver operating characteristic curves of the Quidel Sofia rapid influenza fluorescent immunoassay for detecting influenza A (A) and influenza B (B). HSROC: hierarchical summary receiver-operating characteristic curve; AUC: area under the receiver-operating characteristic curve.
3. Subgroup and sensitivity analyses for the Quidel Sofia rapid influenza FIA to identify influenza A and B
The Higgins I2 statistics proved significant heterogeneity for both the sensitivity (0.91 with 95% CI of 0.88–0.94 for influenza A and 0.87 with 95% CI of 0.82–0.92 for influenza B) (Figure 3) and specificity (0.92 with 95% CI of 0.90–0.95 for influenza A and 0.98 with 95% CI of 0.97–0.98 for influenza B) (Figure 4). Subgroup analyses were also performed to investigate potential sources of heterogeneity (Table 2). The sensitivity of the Quidel Sofia rapid influenza FIA was significantly increased when tests were performed in children (0.86 with 95% CI of 0.78–0.92 for influenza A and 0.79 with 95% CI of 0.71–0.85 for influenza B) than when tests were performed in adults (0.74 with 95% CI of 0.67–0.79 for influenza A and 0.33 with 95% CI of 0.10–0.65 for influenza B).
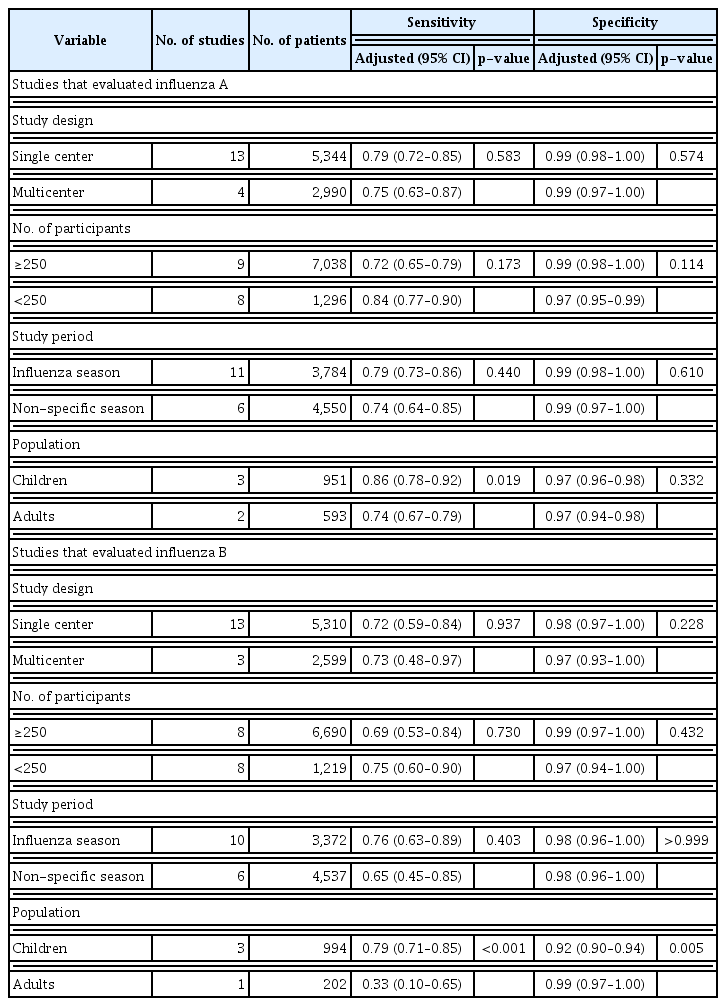
Subgroup analysis for the diagnostic performance of the Quidel Sofia rapid influenza fluorescent immunoassay
In sensitivity analysis to investigate the influence of each individual study on the overall analysis estimate, one study had a significantly different sensitivity than other studies on influenza A24. Even after exclusion of that study24, the pooled sensitivity across studies on influenza A was similar to that of overall studies (0.79 with 95% CI of 0.75–0.82). Instead, the heterogeneity decreased (0.51 with 95% CI of 0.23–0.79).
Discussion
According to the rule set by the Food and Drug Administration (FDA), RIDTs for influenza A and B are required to have a sensitivity of at least 80% and a specificity of at least 95% compared to an FDA-cleared nucleic acid based-test or other currently appropriate tests and FDA accepted comparator methods other than a correctly performed viral culture method28. A recent systematic review and meta-analysis performed a search up to May 2017 and compared accuracies of traditional RIDTs, rapid NAATs, and DIAs in patients with suspected influenza5. For diagnosis of influenza A and B, pooled sensitivities of DIAs and the Quidel Sofia rapid influenza FIA were 80.0% and 76.8%, respectively5. In the present study, compared to RT-PCR, the pooled sensitivity of the Quidel Sofia rapid influenza FIA to identify influenza A and B were 78% and 72%, respectively. Our findings did not quite reach the target level of sensitivity required by the FDA. Therefore, some patients with negative results on the Quidel Sofia rapid influenza FIA might still need be confirmed to have an influenza infection by an alternative diagnostic method that is more sensitive.
Influenza type could affect the accuracy of RIDTs. A previous meta-analysis has revealed that overall RIDTs show an increased sensitivity for detecting influenza A than for detecting influenza B (64.6% vs. 52.2%; p=0.05)29. Influenza A virus can cause more severe disease, higher influenza-associated hospitalization, and more death than influenza B virus29. Higher virulence of influenza A might lead to higher viral burden, which can result in a relatively higher sensitivity19. In the present study, although the pooled sensitivity of the Quidel Sofia rapid influenza FIA for identifying influenza A tended to be higher than that for identifying influenza B, the difference between the two was not statistically significant.
The type of specimen might also lead to difference in diagnostic performances. Although we tried to perform subgroup analyses according to specimens, we could only find two suitable studies26,27. One study compared the results of testing throat and NP swabs specimens26. The sensitivity for each type of specimens was 72% and 100% for detecting influenza A and 71% and 100% for detecting influenza B, respectively26. In the other study, sensitivities of the Quidel Sofia rapid influenza FIA with saliva specimens were comparable to those with NP swabs specimens27. The sensitivity of the test with NP swabs samples was significantly higher than that with saliva samples for detecting influenza A (74.2% vs. 59.2%, p=0.014) and similar between two samples in influenza B (75.9% vs. 65.5%, p=0.387)27. Based on results of these two studies regarding different specimens used for detection, the sensitivity of the Quidel Sofia rapid influenza might be lower for detecting influenza A and B viruses using oropharynx or saliva samples.
The Quidel Sofia rapid influenza FIA has advantages of being simple, fast, and easy for viral testing. The pooled specificity of this tool in our study was approximately 98%, above the target level for detecting both influenza A and B. From these findings, we believe that clinicians could diagnose influenza with assurance on the basis of a positive result from the Quidel Sofia rapid influenza FIA.
Large heterogeneities are commonly reported for systematic reviews of studies on diagnostic test accuracy30. Substantial between-study heterogeneity among enrolled studies was also observed in the present study. Age is a probable source of heterogeneity for between-studies in pooled estimates. In the present study, pooled sensitivities of the Quidel Sofia rapid influenza FIA were significantly higher in children (by approximately 12% higher for influenza A and 46% higher for influenza B) than in adults. The duration of influenza virus shedding is commonly measured from the time of symptom onset to the time of shedding cessation. Children have been reported to have a tendency to shed the virus for a longer duration than adults31. Longer duration of influenza virus shedding in children might be associated with a higher sensitivity of this test in children than in adults. However, because the number of studies that distinguished children from adults was very small, our findings should be interpreted with caution.
The sensitivity observed in one study24 was significantly lower than that observed in most studies. In that study, an older patient population (median age of 57 years) and a study protocol that did not specify the need for particular symptoms or duration of illness might have contributed to the significantly lower sensitivity24. These factors might have been affected by low virus shedding24. In our sensitivity analysis conducted after excluding this study24, the between-study heterogeneity was decreased.
To the best of our knowledge, this is the first meta-analysis to investigate the Quidel Sofia rapid influenza FIA for detecting influenza. However, potential limitations of the present study should be considered when interpreting our results. First, because this present study was based on a relatively small number of trials, our results should be carefully interpreted due to its limited statistical power. Second, we could not make an assessment for publication bias since no reliable methods existed to investigate this issue for diagnostic test accuracy studies32, Finally, as a sample for viral diagnosis, nasopharyngeal aspirates could show higher quality than nasopharyngeal swabs. As mentioned previously, although we tried to investigate the diagnostic accuracy of the Quidel Sofia rapid influenza FIA according to the type of samples, we were unable to perform such analysis because of data limitations.
In conclusion, we found that pooled sensitivities of the Quidel Sofia rapid influenza FIA were slightly below the target level set by the FDA for both influenza A and B. Therefore, physicians should consider the possibility of false-negative results by this test, especially for adults. Although pooled specificities of this test was very high for both influenza A and B, substantial between-study heterogeneity requires careful interpretation of the data.
Notes
Authors’ Contributions
Conceptualization: Lee J. Methodology: Lee J, Song JU. Formal analysis: Lee J, Song JU. Data curation: Lee J, Song JU. Investigation: Lee J, Song JU. Writing - original draft preparation: Lee J, Song JU. Writing - review and editing: Lee J, Kim YH. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a research grant from the Jeju National University Hospital Research Fund of Jeju National University in 2020.