 |
 |
| Tuberc Respir Dis > Volume 84(2); 2021 > Article |
|
Abstract
Background
Methods
Results
Supplementary Material
Acknowledgments
Notes
Initial data were presented at the 23rd Congress of Asian Pacific Society of Respirology, November 29-December 2 2018, Taipei, Taiwan and 126th Fall Congress of Korean Academy of Tuberculosis and Respiratory Disease (KATRD), November 8-9, 2018, Seoul, Korea.
Authors’ Contributions
Conceptualization: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Methodology: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Formal analysis: Moon M. Investigation: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Jung KS. Writing - review and editing: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Approval of final manuscript: all authors.
Conflicts of Interest
Rhee CK received consulting/lecture fees from MSD, Astra-Zeneca, GSK, Novartis, Takeda, Mundipharma, Boehringer-Ingelheim, Teva, and Bayer. Lee SH, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY and Jung KS report no conflict for interest in relation to this work. Moon M is an employee of Novartis. Jung KS serves as editor-in-chief of the Tuberculosis of Respiratory Diseases, but has no role in the decision to publish this article.
Figure 1
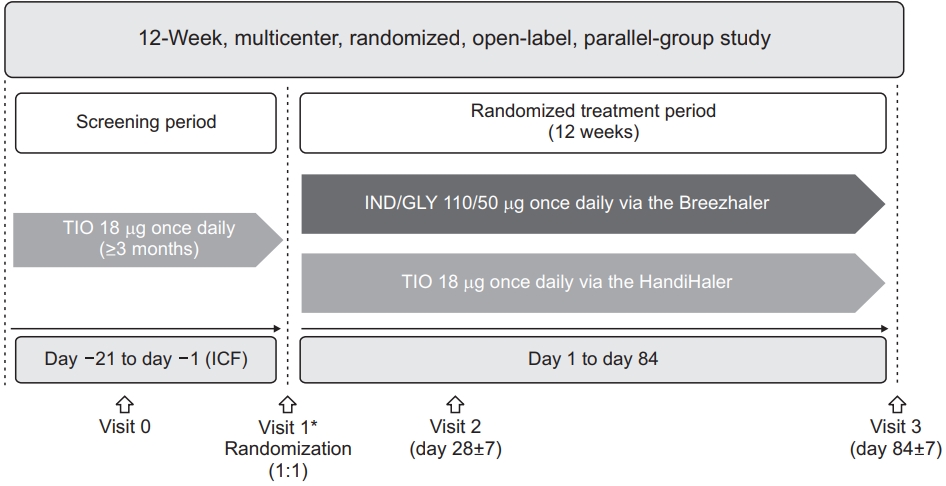
Figure 3
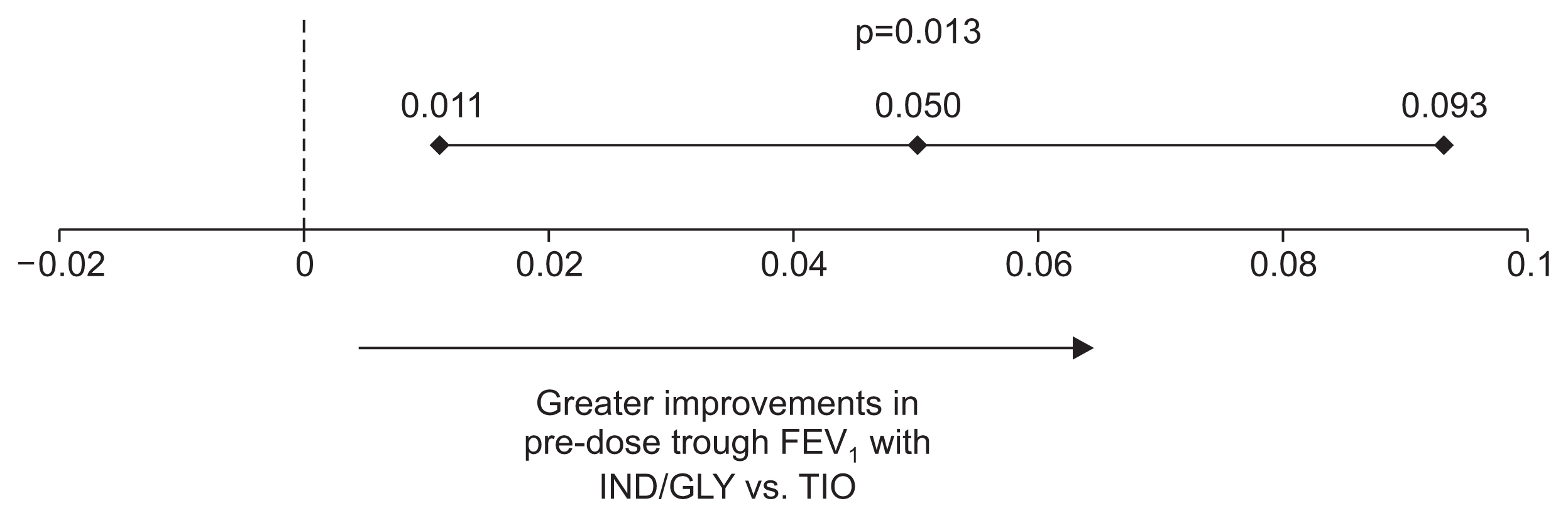
Figure 4
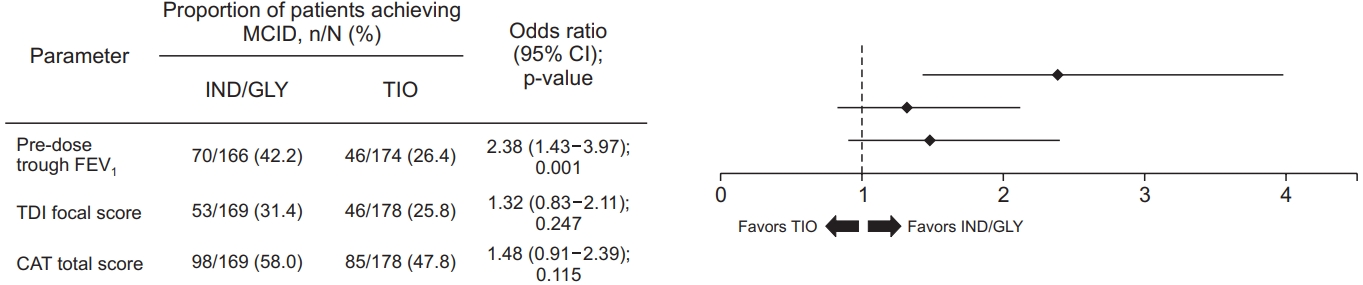
Table 1
Table 2
References
- TOOLS
-
METRICS

- ORCID iDs
-
Sang Haak Lee

https://orcid.org/0000-0001-6259-7656Ki-Suck Jung

https://orcid.org/0000-0002-6878-6543 - Funding Information
-
Novartis Korea Ltd.
- Related articles
-
Economic Burden of Chronic Obstructive Pulmonary Disease: A Systematic Review
Proposed Etiotypes for Chronic Obstructive Pulmonary Disease: Controversial Issues



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Supplement1
Supplement1 Print
Print Download Citation
Download Citation



