 |
 |
| Tuberc Respir Dis > Volume 78(3); 2015 > Article |
|
Abstract
Background
Viridans streptococci (VS) are a large group of streptococcal bacteria that are causative agents of community-acquired respiratory tract infection. However, data regarding their clinical characteristics are limited. The purpose of the present study was to investigate the clinical and radiologic features of community-acquired pneumonia (CAP) with or without parapneumonic effusion caused by VS.
Methods
Of 455 consecutive CAP patients with or without parapneumonic effusion, VS were isolated from the blood or pleural fluid in 27 (VS group, 5.9%) patients. Streptococcus pneumoniae was identified as a single etiologic agent in 70 (control group) patients. We compared various clinical parameters between the VS group and the control group.
Results
In univariate analysis, the VS group was characterized by more frequent complicated parapneumonic effusion or empyema and bed-ridden status, lower incidences of productive cough, elevated procalcitonin (>0.5 ng/mL), lower age-adjusted Charlson comorbidity index score, and more frequent ground glass opacity (GGO) or consolidation on computed tomography (CT) scans. Multivariate analysis demonstrated that complicated parapneumonic effusion or empyema, productive cough, bed-ridden status, and GGO or consolidation on CT scans were independent predictors of community-acquired respiratory tract infection caused by VS.
Conclusion
CAP caused by VS commonly presents as complicated parapneumonic effusion or empyema. It is characterized by less frequent productive cough, more frequent bed-ridden status, and less common CT pulmonary parenchymal lesions. However, its treatment outcome and clinical course are similar to those of pneumococcal pneumonia.
Viridans streptococci (VS) are a heterogeneous group of streptococci that remain when β-hemolytic streptococci, enterococci, and pneumococci are excluded1. VS, part of the normal microbial flora of humans, are most prevalent in the oral cavity and upper respiratory tract, followed by the gastrointestinal tract and female genital tract, particularly the vagina2,3. VS are generally considered harmless commensals but their introduction to sterile sites outside of normal habitats can cause severe infections, such as endocarditis4. Although VS were previously thought to rarely cause community-acquired pneumonia (CAP)5, they have emerged as a causative agent of lung abscess and empyema in several case series3,6,7,8. In recent studies, VS were reported to be the main etiologic agent of empyema9,10 and lung abscess11,12,13.
Community-acquired respiratory tract infection caused by VS is not as rare as previously believed; VS accounted for 3%-15% of CAP in a few studies8,13. However, data are sparse regarding the clinical and radiologic features of community-acquired VS pneumonia with or without parapneumonic effusion. The aim of the present study was to investigate the clinical characteristics of CAP by comparing the clinical and computed tomography (CT) parameters between patients with CAP caused by VS and those with pneumococcal pneumonia.
This study included patients fulfilling the following criteria: (1) a new radiographic infiltrate and two or more of symptoms or signs (cough, sputum, dyspnea, fever, pleuritic chest pain, crackles, or rhonchi); or (2) complicated parapneumonic effusion or empyema.
Complicated parapneumonic effusion or empyema was diagnosed if the parapneumonic effusion fulfilled one of the following criteria: (1) pleural fluid pH <7.2; (2) pleural fluid glucose <60 mg/dL; (3) microorganisms identified by Gram stain or cultured from pleural fluid; and (4) presence of frank pus in the pleural space. Patients with hospital-acquired pneumonia14, healthcare-associated pneumonia14, an active thoracic malignancy, or immunosuppression were excluded.
The present study was retrospectively performed, and a total of 933 patients with CAP with or without parapneumonic effusion were hospitalized and treated at Kyungpook National University Hospital (KNUH), a tertiary referral center, in Daegu, South Korea, between January 2011 and October 2013. Regardless of the presence or absence of parapneumonic effusion, the VS group included CAP patients with blood culture or pleural fluid positive for VS. We selected CAP patients due to Streptococcus pneumoniae as the control group. Pneumococcal pneumonia was diagnosed based on a positive urinary pneumococcal antigen test result or isolation of S. pneumoniae from the sputum, blood, or pleural fluid samples. Patients with mixed infections were excluded from the control group. This study was approved by the Institutional Review Board of the KNUH (2013-12-040), which waived the requirement for written informed consent because of the retrospective nature of the study.
Demographic data including age, gender, smoking history, heavy drinking, and body-mass index (BMI) were reviewed. Heavy drinking was defined as the consumption of seven or more drinks on one occasion for men and five or more drinks for women15. Risk factors for aspiration included impaired swallowing due to neurologic disease, mechanical obstruction or esophageal dysfunction, impaired consciousness, and history of vomiting or witnessed aspiration, as previously defined16. Bed-ridden status and comorbid conditions were checked, and Charlson comorbidity index (CCI) scores were calculated17. The presence of respiratory symptoms, altered mental status, and vital signs were recorded. The severity of pneumonia at initial presentation was defined according to the pneumonia severity index (PSI)18 and CURB-6519 score. Clinical course, measured by hospital stay, treatment success, in-hospital mortality, and use of mechanical ventilation or inotropic support were reviewed. Treatment success was defined as cases with clinically and radiologically improved status. Lastly, complicated pleural effusion or empyema and pleural drainage with percutaneous catheter or chest tube were included in the parameters.
Blood laboratory parameters including white blood cell count, erythrocyte sedimentation rate, and levels of C-reactive protein, procalcitonin, N-terminal-pro-B-type-natriuretic peptide, troponin I, sodium, albumin, and blood urea nitrogen were compared between the two groups.
CT scans were reviewed by one radiologist and one chest physician and a consensus diagnosis was reached. We checked for consolidation, ground glass opacity (GGO), centrilobular nodules, bronchial wall thickening, cavity, and pleural effusion. Similar to previous studies20,21, patients were classified into four levels by measuring the maximum fluid thickness between the visceral and parietal pleurae on a CT scan: no pleural effusion; small pleural effusion, fluid thickness of 5-20 mm; moderate pleural effusion, fluid thickness of 21-50 mm; and large pleural effusion, fluid thickness >50 mm.
Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as medians with interquartile ranges (IQR) for continuous variables and as numbers with percentages for categorical variables. The Mann-Whitney U test was used to compare continuous variables between the VS and control groups, and chi-squared test or Fisher exact test was used to compare categorical variables. Multivariate logistic regression analysis was used to identify predictors of CAP caused by VS with or without parapneumonic effusion. The Hosmer-Lemeshow test was used as a test to assess the goodness-of-fit of logistic regression models. p-values of <0.05 were considered statistically significant.
Causative microbes were identified in 455 from a total of 933 patients diagnosed with CAP with or without parapneumonic effusion. VS were isolated in 27 patients (5.9%), and S. pneumoniae were identified in 97 patients, 27 of whom were excluded from analysis owing to the presence of mixed infections. S. mitis/oralis were the most common VS species, followed by S. sanguis and S. constellatus (Figure 1). Only one VS species was isolated from each patient, except one patient in whom S. mitis/oralis and S. parasanguinis were cultured from the blood samples. We compared various parameters between the VS (n=27) and control groups (n=70).
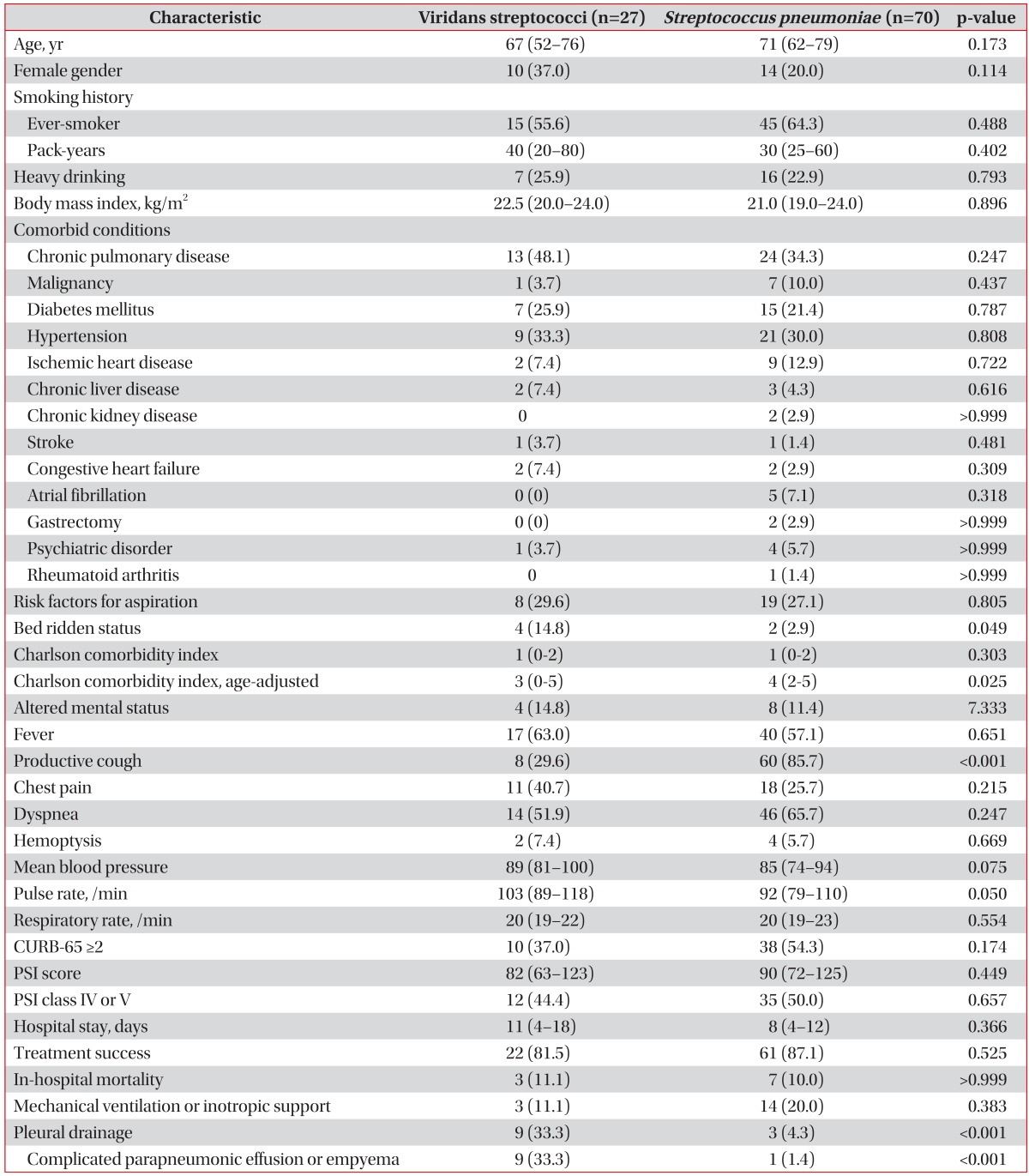
Age, male gender, smoking history, BMI, and heavy drinking did not significantly differ between the VS and control groups (Table 1). There were no significant differences in CCI between the two groups, but the VS group had a significantly lower age-adjusted CCI score than the control group. The frequency of bed-ridden status was significantly higher in the VS group than in the control group. The incidence of risk factors for aspiration was not significantly different between the two groups.
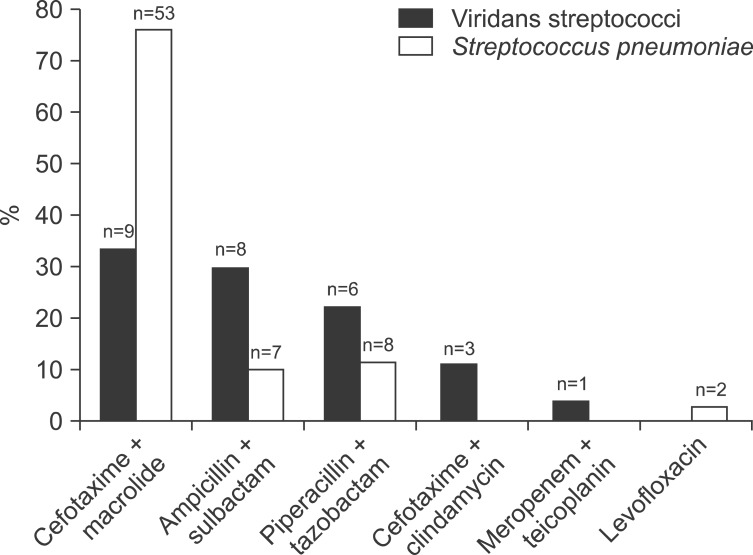
Productive cough was significantly less common in the VS group than in the control group. Complicated parapneumonic effusion or empyema occurred more frequently in the VS group than in the control group. The PSI and CURB-65 scores did not significantly differ between the two groups. No differences were observed in clinical course parameters between the two groups, including hospital stay, success rate, and inhospital mortality. Overall, the median duration of follow-up was 46 days (IQR, 12-113 days). The most commonly used antibiotic regimen in the two groups was cefotaxime or ceftriaxone plus macrolide or fluoroquinolone (Figure 2).
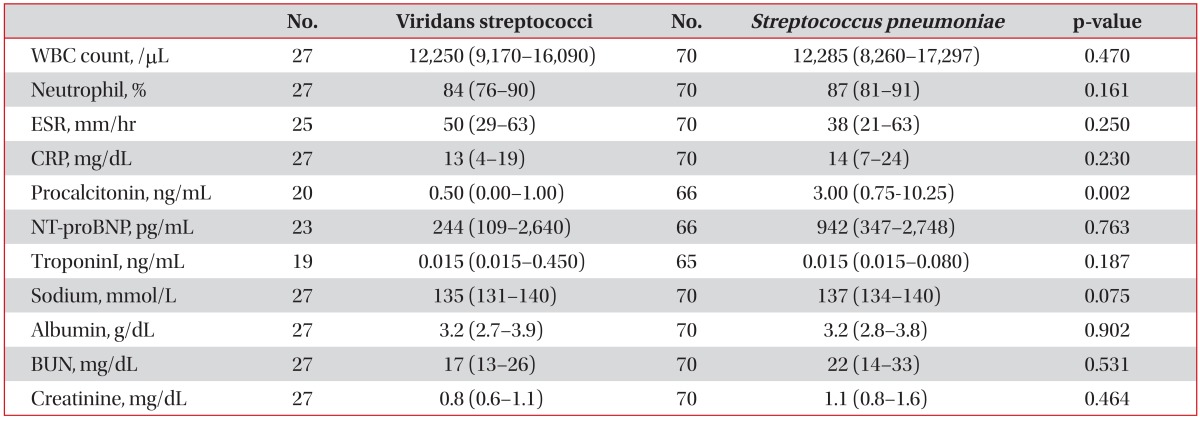
Laboratory findings are summarized in Table 2. Procalcitonin level was significantly lower in the VS group than in the control group. The remaining parameters did not significantly differ between the two groups.
CT findings are presented in Table 3. Pleural effusion was more frequently observed in the VS group than in the control group. In contrast, pulmonary parenchymal lesions including consolidation and GGO were less commonly observed in the VS group.
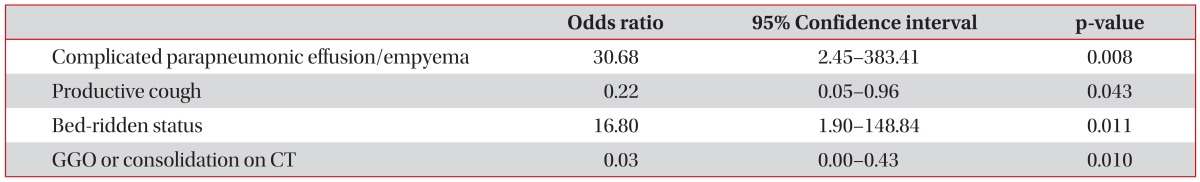
Parameters significant by univariate analysis were selected as candidate predictors for multivariate analysis: complicated parapneumonic effusion or empyema, productive cough, elevated procalcitonin (>0.5 ng/mL), bed-ridden status, age-adjusted CCI score, and GGO or consolidation on CT scans. The Hosmer-Lemeshow test indicated that the overall model fit was good (p=0.699). Multivariate analysis showed that complicated parapneumonic effusion or empyema, productive cough, bed-ridden status, and GGO or consolidation on CT scans were independent predictors of community-acquired respiratory tract infection caused by VS (Table 4).
The present study showed that, although uncommon, VS may be a cause of community-acquired respiratory infection. Compared with pneumococcal pneumonia, respiratory tract infection due to VS was characterized by less frequent productive cough and more frequent bed-ridden status. One-third of patients presented with complicated parapneumonic effusion or empyema. However, treatment outcome and prognosis of VS pneumonia did not differ from those of pneumococcal pneumonia.
Similar to previous studies8,13, we found VS to be the causative agent in 5.9% of CAP cases, suggesting that pneumonia caused by VS might not be as rare as previously reported. VS are normal microbial flora of the mouth, upper respiratory tract, gastrointestinal tract, and vagina3. VS can cause respiratory tract infection through the following routes: (1) aspiration of oral secretions; (2) direct implantation due to trauma or surgery; (3) by extension from contiguous foci of infection; and (4) via the bloodstream from distant sites7. In contrast to a previous study9, no patients in this study had a history of trauma or surgery, which excludes direct implantation as a likely pathogenic route. No contiguous foci of infection were detected on CT scans of 25 patients whose CT scans were available. Although only six patients from the VS group underwent echocardiography, multiple nodules or multifocal infiltrates suggestive of septic pulmonary embolism22 were not observed. Consequently, aspiration of oral secretions is the most plausible access route of VS into the respiratory tract. Furthermore, a previous study reported mixed infections comprising anaerobes and members of S. milleri group to be common in thoracic infections, especially lung abscess and empyema8. These results suggest that respiratory tract infection caused by VS may commonly present with aspiration pneumonia. However, there was no difference in aspiration risk factors between the VS and S. pneumoniae groups in the present study, which corresponds to a previous study where only a half of patients with S. milleri pulmonary infections were predisposed to aspiration8.
VS are one of the main etiologic agents of empyema. In the same context, we found that two-thirds of patients with VS infection had pleural effusion and one-third presented with complicated pleural effusion or empyema. This is a major feature that distinguishes VS infection from pneumococcal pneumonia. By contrast, pulmonary parenchymal lesions, such as consolidation and GGO, were less frequently observed in patients with VS infection. Similarly, productive cough occurred less commonly in the VS group than in the S. pneumoniae group. Serum procalcitonin elevation reflects severe bacterial infection, in particular pneumonia23. Serum procalcitonin level was significantly lower in the VS group, although the p-value did not reach a statistical significance in multivariate analysis. This finding was in line with lower PSI and CURB-65 score in the VS group despite no statistical significance, suggesting that VS might cause less severe infection compared with S. pneumoniae.
Like pneumococcal pneumonia, most patients with VS pneumonia were successfully treated with antibiotics with or without pleural drainage. In addition, there were no differences in in-hospital mortality, hospital stay, and mechanical ventilation or inotropic support between the two groups. These results can be explained by the fact that all VS isolates were susceptible to commonly used antibiotics, such as cefotaxime (data not shown).
The present study has some limitations. Due to its retrospective nature, the potential for selection bias should be noted. However, to minimize this potential, all consecutive patients were enrolled in the study. Because VS pneumonia was diagnosed only on the basis of positive blood or pleural fluid cultures, the proportion of patients with VS pneumonia might be underestimated and the possibility that more severe form of VS pneumonia was chosen could not be excluded. As VS is also a common pathogen causing infective endocarditis, this diagnosis required exclusion. Of 21 patients from whom VS were cultured only using blood samples, six underwent echocardiography, which showed no evidence of endocarditis. However, the remaining patients had typical CT findings suggestive of lobar pneumonia or bronchopneumonia. Thus, the possibility of endocarditis was considered to be low.
In conclusion, CAP caused by VS commonly presents with complicated parapneumonic effusion or empyema. It is characterized by less frequent productive cough, more frequent bed-ridden status, and less common pulmonary parenchymal lesions on CT scans. However, its clinical course and treatment outcome are similar to those of pneumococcal pneumonia.
References
1. Doern CD, Burnham CA. It's not easy being green: the viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol 2010;48:3829-3835. PMID: 20810781.



2. Tunkel AR, Sepkowitz KA. Infections caused by viridans streptococci in patients with neutropenia. Clin Infect Dis 2002;34:1524-1529. PMID: 12015700.



3. Waitkins SA, Ratcliffe JG, Roberts C. Streptococcus milleri found in pulmonary empyemas and abscesses. J Clin Pathol 1985;38:716-717. PMID: 4008669.

4. Sugihara E, Kido Y, Okamoto M, Koyanagi T, Niizeki T, Hirota N, et al. Clinical features of acute respiratory infections associated with the Streptococcus milleri group in the elderly. Kurume Med J 2004;51:53-57. PMID: 15150900.


5. Sarkar TK, Murarka RS, Gilardi GL. Primary Streptococcus viridans pneumonia. Chest 1989;96:831-834. PMID: 2791680.


6. Molina JM, Leport C, Bure A, Wolff M, Michon C, Vilde JL. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand J Infect Dis 1991;23:659-666. PMID: 1815325.


7. Hocken DB, Dussek JE. Streptococcus milleri as a cause of pleural empyema. Thorax 1985;40:626-628. PMID: 4035635.



8. Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis 1995;21(Suppl 3):S238-S243. PMID: 8749672.



9. Porta G, Rodriguez-Carballeira M, Gomez L, Salavert M, Freixas N, Xercavins M, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J 1998;12:357-362. PMID: 9727785.


10. Jerng JS, Hsueh PR, Teng LJ, Lee LN, Yang PC, Luh KT. Empyema thoracis and lung abscess caused by viridans streptococci. Am J Respir Crit Care Med 1997;156:1508-1514. PMID: 9372668.


11. Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis 2005;40:915-922. PMID: 15824979.



12. Takayanagi N, Kagiyama N, Ishiguro T, Tokunaga D, Sugita Y. Etiology and outcome of community-acquired lung abscess. Respiration 2010;80:98-105. PMID: 20389050.


13. Ishida T, Hashimoto T, Arita M, Ito I, Osawa M. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest 1998;114:1588-1593. PMID: 9872193.


14. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. PMID: 15699079.


15. Oh MG, Han MA, Park J, Ryu SY, Park CY, Choi SW. Health behaviors of cancer survivors: the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV, 2007-09). Jpn J Clin Oncol 2013;43:981-987. PMID: 23975890.



16. Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis 2011;53:107-113. PMID: 21690616.



17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-383. PMID: 3558716.


18. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-250. PMID: 8995086.


19. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-382. PMID: 12728155.



20. Cha SI, Shin KM, Jeon KN, Yoo SS, Lee J, Lee SY, et al. Clinical relevance and characteristics of pleural effusion in patients with Mycoplasma pneumoniae pneumonia. Scand J Infect Dis 2012;44:793-797. PMID: 22681452.


21. Luo YF, Robbins IM, Karatas M, Brixey AG, Rice TW, Light RW. Frequency of pleural effusions in patients with pulmonary arterial hypertension associated with connective tissue diseases. Chest 2011;140:42-47. PMID: 21212140.


22. Lee SJ, Cha SI, Kim CH, Park JY, Jung TH, Jeon KN, et al. Septic pulmonary embolism in Korea: Microbiology, clinicoradiologic features, and treatment outcome. J Infect 2007;54:230-234. PMID: 16750858.


23. Christ-Crain M, Muller B. Procalcitonin in bacterial infections: hype, hope, more or less? Swiss Med Wkly 2005;135:451-460. PMID: 16208582.

Figure 1
Viridans streptococcal isolates. Streptococcus mitis/oralis is the most common pathogen, followed by S. sanguis and S. constellatus (%, the number of each isolate per the number of total viridans streptococcal isolates): S. mitis/oralis is isolated from blood (n=8) and pleural fluid (n=2); S. sanguis is from blood (n=6); S. constellatus is from blood (n=2) and pleural fluid (n=2); S. intermedius is from pleural fluid (n=3) and blood (n=1); S. salivarius, S. parasanguinis, and S. anginosus is from blood (n=2, respectively).

Figure 2
Antibiotic regimens for community-acquired pneumonia. Each regimen includes the following antibiotics: cefotaxime+macrolide: cefotaxime (or ceftriaxone) plus macrolide (or fluoroquinolone); ampicillin+sulbactam: ampicillin+sulbactam with or without macrolide; piperacillin+tazobactam: piperacillin+tazobactam (piperacillin+sulbactam, ticarcillin+clavulanic acid, or maxipime) with or without fluoroquinolone (or macrolide); cefotaxime+clindamycin: cefotaxime plus clindamycin; meropenem+teicoplanin: meropenem plus teicoplanin (or vancomycin); levofloxacin: levofloxacin (or moxifloxacin).
- TOOLS
-
METRICS

- Related articles
-
A case of community-acquired acinetobacter calcoaceticus pneumonia.1991 March;38(1)
Clinical characteristics of synchronous multiple primary lung cancer.1991 September;38(3)
Clincal Manifestations of Patients Dying of Severe Community Acquired Pneumonia.1994 October;41(5)
Clinical Characteristics of Pulmonary Aspergillosis.1994 December;41(6)
The Clinical Characteristics of Mycoplasmal Pneumonia in Adults.1995 April;42(2)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



