Risk Factors for Mortality in Hospitalized Patients with COVID-19: An Overview in a Mexican Population
Article information
Abstract
Background
Currently, Mexico ranks third worldwide in mortality due to coronavirus disease pandemic 2019 (COVID-19) and reliable information is scarce, with the available data focused on epidemiological characteristics. This study aimed to identify the risk factors associated with mortality and outcomes in hospitalized Mexican patients with COVID-19.
Methods
We prospectively assessed patients admitted to a COVID-19 reference center in southeast Mexico between March 28 and June 30, 2020. Mortality was defined as survivors or non-survivors and univariate and multivariate logistic regression analyses were performed to explore the association of the clinical characteristics and laboratory parameters with mortality.
Results
We included 200 patients with a mean age of 55 years, 69% were men and 72% had at least one chronic comorbidity. Eighty-six patients required invasive mechanical ventilation (IMV) with an overall mortality rate of 82.5%. Only 51% of the patients with IMV were admitted to the intensive care unit (ICU), with a survival rate of 27.3%, but only 7.2% for patients without ICU admissions (p=0.014). The multivariate analysis found that a neutrophil-to-lymphocyte ratio ≥9 (odds ratio [OR], 4.64; 95% confidence interval [CI], 2.05–10.53) albumin <3.5 g/dL (OR, 3.76; 95% CI, 1.56–9.07), lactate dehydrogenase (LDH) level ≥725 U/L (OR, 5.45; 95% CI, 2.36–12.57), and IMV (OR, 64.7; 95% CI, 15.20–275.39) were independent risk factors associated with mortality.
Conclusion
Neutrophil-to-lymphocyte ratio, LDH, albumin, and IMV were independent risk factors for mortality in Mexican patients with COVID-19. Also, the availability of ICU resources is invaluable for better outcomes in critically ill patients. Our results could provide clinical information for timely decision-making in low-and-middle income countries to overcome the pandemic.
Introduction
The coronavirus disease pandemic of 2019 (COVID-19) continues to spread globally, causing in the deaths of more than half a million people worldwide and unprecedented economic impact. So far, it has been recognized that advanced age, chronic diseases [1-3], and several laboratory result abnormalities [4] are associated with severe and fatal forms of COVID-19. Current pandemic concerns are centered in The Americas where, according to a report by Johns Hopkins University [5] together with the World Health Organization (WHO), the United States, Brazil, and Mexico have ranked among the top five countries in the world since July for the highest number of confirmed COVID-19 deaths. Currently, Mexico holds the third place for COVID-19 deaths worldwide [6].
Mexico’s adult population has a higher prevalence of several chronic medical conditions including overweight status and obesity (72.5%) [7], hypertension (25.5%) [8], and diabetes mellitus (13.7%) [9]. Previous studies have identified these chronic medical conditions as risk factors associated with severe disease and mortality in COVID-19 patients [10].
Since early July, Mexico has faced the epidemiological phase of greatest community transmission as the nation’s economic activity resumed, resulting in a significant increase in the number of daily cases and tripling COVID-19 mortality in our territory [11]. Reliable information on our population is scant and available data has focused only on epidemiological characteristics. There is an urgent need for clinical data to support decision-making in the medical management and availability of intensive care unit (ICU) resources related to the high number of deaths.
The available information of the mortality risk factors and outcomes in hospitalized patients with COVID-19 in the Mexican population so far is scarce. To address this knowledge gap, we conducted a study that examined the clinical characteristics and laboratory parameters to analyzed the mortality risk factors and outcomes the the first 200 patients admitted to a COVID-19 hospital reference center in southeast Mexico.
Materials and Methods
1. Study design and patients
This single-center, observational, ambispective cohort study was conducted at the Hospital Regional de Alta Especialidad de la Peninsula de Yucatan, a third-level reference center currently designated to evaluate and treat patients with COVID-19. All adult patients who were admitted with an acute respiratory illness (fever, cough, dyspnea) and were diagnosed with COVID-19 based on the WHO interim guidance from March 28 to June 30, 2020 were included in our study [12]. The protocol was approved by the local research ethics committee (2020-023). All patients signed informed consent on admission and the information was kept strictly confidential under the guidelines established in the Declaration of Helsinki [13].
2. Data collection
The information of all patients, including demographics, clinical presentation, laboratory parameters and patient outcomes were extracted from medical records. Two researchers independently reviewed the information to double-check the collected data. If data were missing or unclear, the attending physicians were interviewed for clarification. We collected data on age, sex, medical history, and symptoms from onset to hospital admission. Laboratory assessments consisted of a complete blood count, blood chemistry analysis, coagulation profile, D-dimer, assessment of liver, renal and cardiac function, electrolyte measurements, lactate dehydrogenase, creatine kinase (CK), CK-MB, troponin T, and inflammatory biomarkers, as C-reactive protein, erythrocyte sedimentation rate, procalcitonin, and serum ferritin. Laboratory parameters were divided into three categories: hematologic and inflammatory biomarkers, coagulation factors and organ-injury biochemical biomarkers [4]. The primary outcome was mortality defined as survivor or non-survivor at the time of the analysis. Independent predicted factors associated with fatal outcomes were analyzed as secondary outcomes.
On admission, nasal and pharyngeal swabs specimens were sent to the State Public Health Laboratory for processing (Laboratorio Estatal de Referencia Epidemiologica del Estado de Yucatan). All specimens were tested for COVID-19 with real-time reverse-transcriptase-polymerase chain reaction based on the protocol established by the WHO [14,15].
3. Statistical analysis
We expressed descriptive data as median (interquartile range, IQR) for continuous variables and number (%) for categorical variables. We assessed differences between non-survivors and survivors using Wilcoxon’s rank-sum test for continuous variables and chi-square test or Fisher exact test for categorical variables. Univariate and multivariate logistic regression analysis were performed to explore the association of clinical characteristics and laboratory parameters (inflammation-related indices and organ-injured related biomarkers) with mortality (dependent variable). Tests were two-sided with significance set at α less than 5%. The STATA version 13 software (Stata Corp., College Station, TX, USA) was applied for all analysis.
Results
1. Baseline characteristics of the study population
We included the first 200 cases with complete data who were followed up and had an outcome until June 30, 2020. Descriptive characteristics are detailed in Table 1. The median age was 55 years (IQR, 41–65), 55% of whom were older than 65 years and 138 (69%) were male. In our cohort, 144 patients (72%) have one or more chronic medical conditions, obesity being the most prevalent (52%), followed by hypertension (30%) and diabetes mellitus (28%). The median duration from symptoms onset to baseline evaluation was 8 days (IQR, 6–9). On admission, most common symptoms were fever (96%), dyspnea (93%), and cough (90%), with oxygen saturation 87% on room air (IQR, 78%–90%), pulse oximetric saturation/fraction of inspired oxygen (SpO2/FiO2) of 414 (371–431), and quick Sequential Organ Failure Assessment (qSOFA) score of 1 point (IQR, 1–1). Overall, the need of invasive mechanical ventilation (IMV) was 43% of all hospitalized patients with an overall mortality rate of 82.5%. Only the 51% of patients with IMV received attention in ICU, 32 patients (72.7%) died in a ICU vs 39 patients (92.7%) who died in a different area of the hospital. The median of hospital stay was 6 days (4–12 days) (Table 1).
2. Clinical and epidemiological differences between non-survivors and survivors
Compared to survivors, non-survivors of COVID-19 represented the 38.7% of the entire cohort with a higher proportion of patients older than 65 years (43% vs. 18%, p<0.001) (Table 1).
There were non-significant differences between groups in chronic medical conditions and symptoms, nonetheless, intensity of dyspnea on admission among non-survivors was higher considering modified Medical Research Council (mMRC) scale (3 vs. 2, p<0.001). Compared to survivors, non-survivors had a significantly higher heart rate (107 vs. 98, p=0.022) and respiratory rate (30 vs. 28, p=0.003), lower baseline SpO2 (73% vs. 89%, p<0.001) and SpO2/FiO2 (348 vs. 424, p<0.001). Also, mortality in non-survivors who did not received attention on an ICU were higher (92.8% vs. 72.7%, p=0.014).
3. Laboratory indices differences between non-survivors and survivors
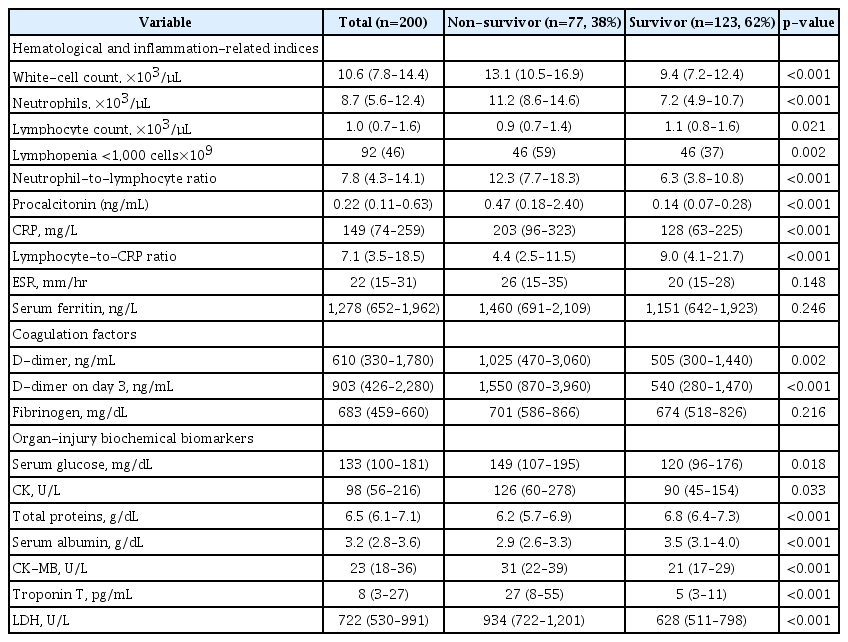
Substantial differences in laboratory findings between non-survivors and survivors are summarized in Table 2. Compared with survivors, levels of hematological and inflammationrelated indices including leukocytosis (13.1 vs. 9.4, p<0.001), neutrophil-to-lymphocyte ratio (NLR; 12.3 vs. 6.3, p<0.001), procalcitonin (0.47 ng/mL vs. 0.14 ng/mL, p<0.001), and C-reactive protein (203 mg/L vs. 128 ng/mL, p<0.001) were significantly higher in the non-survivors group. Also, baseline D-dimer levels were doubled in non-survivors group (1,025 ng/mL vs. 505 ng/mL, p=0.002) and specifically the differences were strengthened on day 3 (1,550 ng/mL vs. 540 ng/mL, p<0.001). Among organ-injury biochemical biomarkers, baseline glucose, CK, CK-MB, high-sensitive troponin T, and lactate dehydrogenase (LDH) were markedly higher in non-survivors (Table 2).
There were non-significant differences between groups in erythrocyte sedimentation rate, serum ferritin nor fibrinogen serum levels. However, in non-survivors group the absolute Lymphocyte Count was lower (0.9 vs. 1.1, p=0.021), as well as the lymphocyte-to-C-reactive protein ratio (4.4 vs. 9.0, p<0.001), total proteins, and serum albumin (2.9 g/dL vs. 3.5 g/dL, p<0.001) than in survivors group (Table 2).
4. Predictors of mortality
Several variables such as age, days from symptom onset to admission, dyspnea mMRC ≥3, respiratory rate, heart rate, SpO2/FiO2, qSOFA score, IMV, hematological and inflammation-related indices and organ-injury biochemical biomarkers were associated with mortality in the univariate logistic regression analysis. However, after multivariate logistic regression, dyspnea mMRC ≥3 (odds ratio [OR], 3.59; 95% confidence interval [CI], 1.51–8.52), SpO2/FiO2 ratio lower than 420 (OR, 6.44; 95% CI, 2.33–17.83), qSOFA score more than 2 points (OR, 3.46; 95% CI, 1.04–11.58) and need for IMV (OR, 64.7; 95% CI, 15.20–275.39) remained as independent risk factors for mortality. Some laboratory biomarkers as NLR ≥9 (OR, 4.64; 95% CI, 2.05–10.53), serum albumin <3.5 g/dL (OR, 3.76; 95% CI, 1.56–9.07), and LDH ≥725 U/L (OR, 5.45; 95% CI, 2.36–12.57) on admission, were independent risk factors associated with mortality in COVID-19 patients (Tables 3, 4).
Discussion
According to a new report by the Pan-American Health Organization (PAHO), the epicenter of the epidemic is currently in countries of America, with more cases reported daily and a higher mortality rate from COVID-19 [16]. As well as in Mexico, many countries in Latin America share a significant prevalence of chronic diseases, thus as emerging economies, there are a considerable number of socially vulnerable groups with economic difficulties in the effective fulfillment of home isolation. The latter, coupled with other socio-economic problems [17,18], converge on the alarming current state of this health emergency. Particularly in Mexico, since the opening of economic activity there has been an increase of 194% in the number of positive cases and mortality by COVID-19 has been tripled [11]. In the absence of specific treatment and an effective vaccine development in the short term, identification and recognition of mortality-related risk factors are considered vitally important for providing timely medical interventions and clinical decision-making on the patient care site.
Our results indicate, as has been consistently reported in previous studies, that age is a significant risk factor for severity and mortality in patients with COVID-19 [19,20]; a cutoff value of 50 years old has been significantly associated with mortality. Certainly, immunosenescence has been involved as an inadequate adaptive immune response leading to poor results [21]. We did not observe differences regarding gender in mortality as had been showed in other cohorts [19,20]. The prevalence of chronic diseases in our population did not allow us to differentiate if any might be considered as a risk factor for mortality since roughly eight out of 10 hospitalized patients have at least one comorbidity. Obesity is a public health problem, occupying the first place in prevalence among the Mexican population, responsible for severe forms of COVID-19 [22,23].
Regarding clinical characteristics of our population admitted with COVID-19, we identified that survivors had more signs and symptoms of the disease, although non-survivors showed a higher intensity in dyspnea measured by the mMRC scale. On physical examination, non-survivors have lower baseline SpO2 with a lower SpO2/FiO2 ratio. This ratio is a well-validated rapid detection index of acute respiratory distress syndrome (ARDS), which correlates with the PaO2/FiO2 calculation where blood gas testing may not be feasible [24]. Therefore, SpO2/FiO2 ratio should be used to early discriminate patients who benefit from aggressive therapy in order to improve oxygenation levels, as more the delay with oxygen support the higher organ damage [25]. A cutoff value of SpO2/FiO2 <420 is reasonable to closely monitor patients and take timely decision-making for admission to ICU’s, since it corresponds to 90% of saturated hemoglobin with a partial oxygen pressure of 60 mm Hg [24-26]. It should be noted that 43% of our population required IMV with an overall mortality rate of 82.5%. This numbers are related with previous reports in countries facing the worse scenario of COVID-19 (Wuhan and New York City) [27,28].
According to serum biomarkers univariate analysis revealed several abnormalities in the non-survivor’s group, some of them are described in previous published reports [29,30]. Nonetheless, in our population the independent variables associated with mortality were NLR, LDH, and serum albumin. The NLR is related with a moderate-to-severe ARDS (NLR >11) [31,32] but also, it is an independent risk factor for intrahospital mortality [32,33]. The cutoff level of NLR in our population was ≥9 and still independently related with mortality. LDH enzyme is a biomarker of severity in many systemic diseases and in patients with COVID-19, increased levels (>445 U/L) have been associated with organic damage and extensive lung injury [29,33]. However, our entire cohort of patients expressed higher values of LDH contrasting to previous reports (≥725 U/L) suggesting either a more severe disease expression or a delay in medical evaluation [33]. Lower levels of serum albumin is an indicator of abnormal liver function and according to the result of a recent meta-analysis, the lower the level of serum albumin the higher risk of a severe or fatal COVID-19 disease due to a delay in viral clearance [4,34].
In addition to the complexity of COVID-19 disease, many challenges are faced in Mexico, including a higher prevalence of chronic diseases, socio-economic factors, the economic reopening and according to WHO guidelines, insufficient hospital infrastructure by population [35], where ICUs represent a limited and invaluable resource for better outcomes in patients. Furthermore, low- and middle-income countries are at risk for an inability to manage an anticipated surge of critically ill COVID-19 patients, even though current literature recommend expanding critical care to guarantee quality of care for these patients. Current estimates suggesting the availability of 0.1 to 2.5 ICU beds per 100,000 population [36]. As well, several studies have shown that among hospitalized patients, 5% to 33% will require admission to an ICU and 75% to 100% of them will require support with IMV [1,27,37]. Mortality of critically ill patients with COVID-19 varies significantly among the published case series, ranging from 26% to 92% [27,28,38]. Supporting this data, in our cohort, we observed that mortality was higher in patients who did not receive a critical support treatment on an ICU. This wide variability can be explained by different case mixes, different organization or availability of ICU’s beds among different countries [28,36-38]. For that reason, COVID-19 represents a massive challenge for health care systems and the ICUs throughout the world and specially in Mexico for being among the low-and middle-income countries [36].
In conclusion, our findings could be usefully for decision-making to improve outcomes and prognosis of patients with COVID-19. Furthermore, may offer a national perspective since the information provided is relevant in order to priorate the needs, especially in low-and-middle income countries (Latin America), where the atmosphere of political disputes, the misinformation and lack of credibility in health authorities make it difficult to set priorities, allocate healthcare resources focusing on equipment and critical care, establish preventive strategies for disease transmission and prevent the collapse of the health system.
Our study has some limitations. First, this study was conducted at a single-center hospital with limited sample size. However, it is a designated center to treat COVID-19. Second, even though we were able to systematically register the information, some specific data might be missed. Third, we were not able to perform blood gas analysis in all patients.
Notes
Authors’ Contributions
Conceptualization: Cortés-Tellés A. Methodology: Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, Ortíz-Farías DL, Núñez-Caamal N, Figueroa-Hurtado E. Formal analysis: Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R. Data curation: López-Romero S, Mancilla-Ceballos R. Investigation: Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, Ortíz-Farías DL, Núñez-Caamal N, Figueroa-Hurtado E. Writingoriginal draft preparation: Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, Ortíz-Farías DL, Núñez-Caamal N, Figueroa-Hurtado E. Writing-review and editing: Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, Ortíz-Farías DL, Núñez-Caamal N, Figueroa-Hurtado E. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Acknowledgements
To ICU staff: Gonzalo Renán Pantoja-Silveira, Irving SosaMarrufo, Yareth Maldonado, Sergio Bonfil, Erik Francisco Romero-Mejía, Freddy Avila, Gerardo Díaz. Internal Medicine staff: Jorge Arturo Valdivieso-Jiménez, Genaro Suárez, Roberto Ambriz Huerta, José Manuel Burgos Borges, Juan Erick Aceves-Díaz. Jesús Antonio Tut-Bojorquez. ICU and general ward nursing staff. X-ray technicians, Respiratory therapists and Nutritionist staff. Dra. Mussaret Zaidi Jacobson for her support in reviewing the manuscript.