Clinical Manifestations and Outcomes of Older Patients with COVID-19: A Comprehensive Review
Article information
Abstract
The consequences of coronavirus disease 2019 (COVID-19) are particularly severe in older adults with a disproportionate number of severe and fatal outcomes. Therefore, this integrative review aimed to provide a comprehensive overview of the clinical characteristics, management approaches, and prognosis of older patients diagnosed with COVID-19. Common clinical presentations in older patients include fever, cough, and dyspnea. Additionally, preexisting comorbidities, especially diabetes and pulmonary and cardiovascular diseases, were frequently observed and associated with adverse outcomes. Management strategies varied, however, early diagnosis, vigilant monitoring, and multidisciplinary care were identified as key factors for enhancing patient outcomes. Nonetheless, the prognosis remains guarded for older patients, with increased rates of hospitalization, mechanical ventilation, and mortality. However, timely therapeutic interventions, especially antiviral and supportive treatments, have demonstrated some efficacy in mitigating the severe consequences in this age group. In conclusion, while older adults remain highly susceptible to severe outcomes from COVID-19, early intervention, rigorous monitoring, and comprehensive care can play a pivotal role in improving their clinical outcomes.
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious illness caused by the severe acute respiratory syndrome coronavirus 2. It was initially detected in Wuhan, China in December 2019, and quickly became a global pandemic [1]. As of July 5, 2023, the World Health Organization (WHO) has reported 767,726,861 confirmed cases of COVID-19 globally, with 6,948,764 deaths [2]. Factors associated with COVID-19 mortality include older age; male sex; underlying health conditions; laboratory biomarkers such as lymphocyte count, serum lactate dehydrogenase (LDH), and C-reactive protein (CRP) levels; and a high viral load during hospitalization [3,4]. Among these factors, older age is well recognized to be associated with increased severity and higher mortality rates [5-9].
As of July 5, 2023, the Republic of Korea has reported 32,256,154 confirmed cases of COVID-19, with 35,071 deaths [10]. According to the Korea Disease Control and Prevention Agency, the percentage of deaths has increased in older age groups (60 to 69 years, 11.36%; 70 to 79 years, 22.69%; ≥80 years, 59.64%) accompanied by higher fatality rates (60 to 69 years, 0.11%; 70 to 79 years 0.43%; ≥80 years, 1.85%) [11]. An age range of 60 to 65 years or older is defined as the criterion for older people associated with poor prognosis in COVID-19 patients. Therefore, this review was conducted to explore the characteristics and prognosis of older patients diagnosed with COVID-19.
This review is crucial for a thorough examination of the clinical manifestations, treatments, and prognostic outcomes in older patients infected with COVID-19. It aims to analyze the ways in which the virus impacts this vulnerable age group, focusing on their symptoms, treatment responses, and factors influencing health outcomes. This in-depth analysis is helpful for improving our understanding of COVID-19’s dynamics in older patients, which is essential for developing more effective clinical strategies and public health policies specifically designed for this high-risk group.
Clinical Features and Common Comorbidities
Symptoms such as fever, cough, dyspnea, myalgia, fatigue, diarrhea, and an asymptomatic presentation can manifest in older patients with COVID-19 [12]. Moreover, the frequency of these symptoms varies across studies. In a study conducted by Li et al. [13], focusing on patients with COVID-19 aged ≥60 years, commonly observed symptoms were fever (78.9%), cough (49%), and dyspnea (31.9%). Similarly, a systematic review and meta-analysis by Singhal et al. [14] on older patients with COVID-19 revealed that fever, cough, and dyspnea were common symptoms. Moreover, when comparing younger and older patients, a higher occurrence of dyspnea [15] and shortness of breath [16] was observed among older patients.
Furthermore, the older patient population has a higher prevalence of multimorbidity, characterized by the coexistence of multiple underlying health conditions [17,18]. The prevalence of one or more comorbidities was approximately 81% among older patients, with hypertension, diabetes, and cardiovascular disease being most common [14]. Similarly, in other studies, older patients had a higher prevalence of hypertension, diabetes, coronary heart disease, and chronic obstructive lung disease [16,19].
Laboratory and Radiologic Findings
Older patients with COVID-19 often have different laboratory findings than younger patients. For example, lymphopenia and leukopenia are frequently observed in older patients [12,14], along with elevated CRP levels [12]. In a study by Wei et al. [20], older patients had higher levels of neutrophils, CRP, aspartate aminotransferase, LDH, glucose, blood urea nitrogen, and creatinine, but lower counts of lymphocytes, hemoglobin, and platelets than younger and middle-aged patients. In study of Ibrahim et al. [21], patients with COVID-19 aged ≥65 years had higher neutrophil counts and lower lymphocyte counts. They also showed increased levels of creatinine, creatine kinase MB, glucose, LDH, bilirubin, D-dimer, and erythrocyte sedimentation rate compared with younger patients. Additionally, study of Liu et al. [22] revealed significant variations in lymphocyte decrease and monocyte increase among different age groups, with leukocytosis and lymphopenia being particularly prominent in older patients.
On radiological evaluation, the predominant manifestations of COVID-19 often involve airspace abnormalities such as consolidations or ground-glass opacities (GGO) [23]. Figure 1 illustrates examples of the radiological findings of COVID-19. These are typically bilateral, situated peripherally, and primarily found in the lower lung fields [23]. Specifically, in older patients, bilateral lung infiltration is the most observed feature [14]. In study of Statsenko et al. [24], increasing age was associated with greater lung involvement on radiologic images, and cases with GGO were more frequently observed. In a study by Sano et al. [25], computed tomography scans of patients with COVID-19 aged ≥75 years predominantly showed non-segmental, peripherally dominant GGO. However, when comparing older and younger patients, some studies have highlighted cases wherein unilateral findings were more prevalent [19] or where imaging results showed no significant age-related differences [26].

Radiologic findings of older patients with coronavirus disease 2019 (COVID-19). (A, B) Normal lung on radiography and computed tomography (CT), (C, D) ground-glass opacities on chest X-ray and CT, (E, F) consolidation on chest Xray and CT, and (G, H) fibrosis on chest X-ray and CT. L: left; R: right; PA: posterior anterior; AP: anterior posterior.
Treatment and Prevention
Older adults [16,27,28], unvaccinated individuals [29,30], and those with certain medical conditions [31-33] face a higher risk of severe COVID-19. Vaccination reduces this risk, and for vaccinated people who are 65 years of age or older or have other risk factors, it may help them get better.
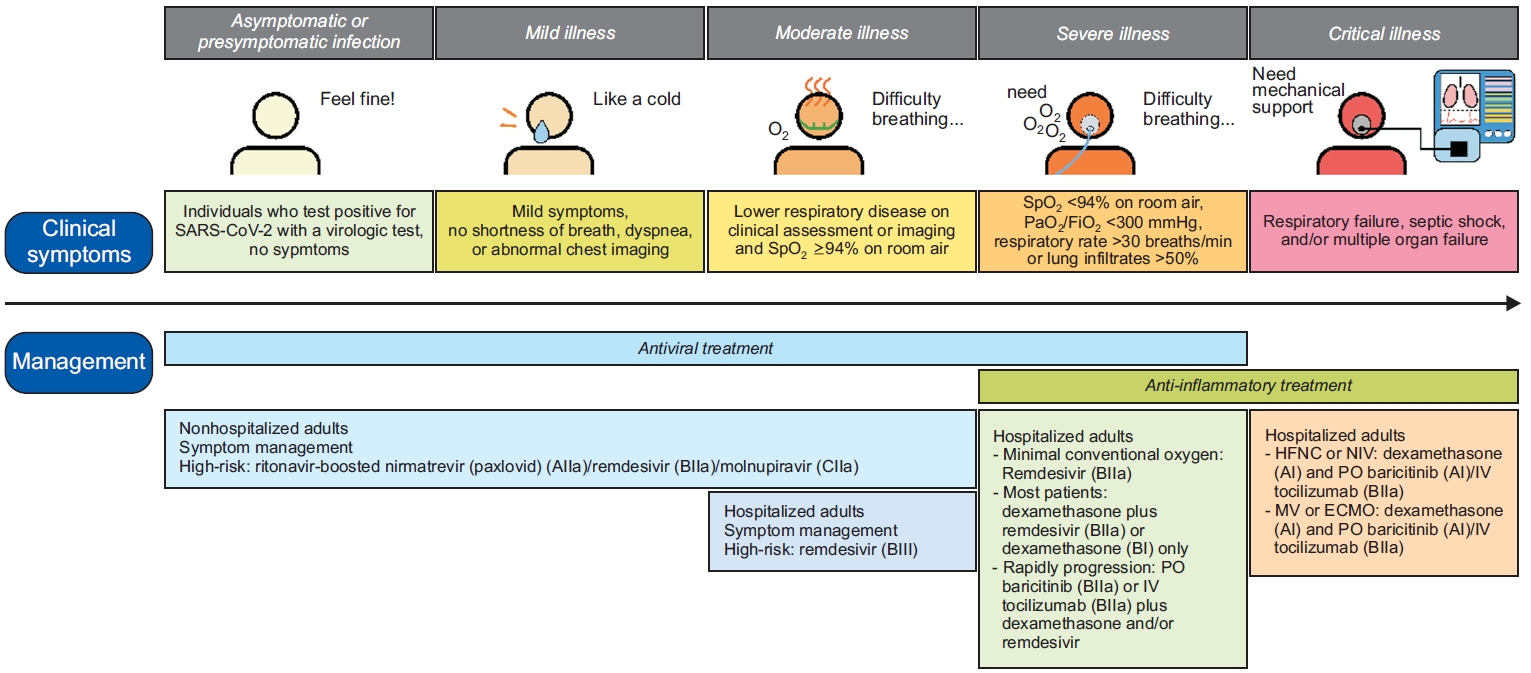
Figure 2 illustrates an overview of the treatment for patients with COVID-19 [34]. In non-hospitalized patients with mild-to-moderate illness, treatment typically involves symptom management combined with the administration of ritonavir-boosted nirmatrelvir (Paxlovid, Pfizer, New York, NY, USA) [35-37] or remdesivir [38,39]. Cases wherein these two drugs are unfeasible, molnupiravir may be considered [35]. In a study [35] involving patients with COVID-19 residing in nursing homes with an average age of 84.8 years, both molnupiravir and paxlovid reduced hospitalizations, intensive care unit admissions, mortality, and the need for invasive mechanical ventilation (MV) compared to patients not treated with antiviral agents. Furthermore, initiating treatment within 5 days of symptom onset appears to offer greater risk reduction.
However, in hospitalized patients, the treatment approach varies according to disease severity. For patients who do not require an oxygen supply, neither dexamethasone nor other systemic corticosteroids are recommended [40]; however, remdesivir may be considered [41]. Moreover, remdesivir can be administered in patients requiring minimal conventional oxygen [41,42], however, most patients requiring conventional oxygen may benefit from either a combination of dexamethasone and remdesivir [42] or dexamethasone monotherapy [40,43]. According to a recent review by the Cochrane Library [41], remdesivir administration in patients with moderate-to-severe COVID-19 resulted in a slight promotion of clinical improvement within 28 days and indicated a potential reduction in the risk of clinical deterioration. On the other hand, the concurrent use of oral baricitinib [44,45] or intravenous tocilizumab [46] should be explored in rapidly deteriorating patients. For those requiring interventions such as high-flow nasal cannula, non-invasive ventilation, MV, or extracorporeal membrane oxygenation (ECMO), the addition of oral baricitinib [44,47] or intravenous tocilizumab [46,48] to dexamethasone [40,43] is feasible. When administered to older patients, both tocilizumab [48] and baricitinib [47] have been observed to reduce the risk of mortality. In a study [40] on inpatients with COVID-19 with a median age of 64 years, the use of dexamethasone reduced all-cause mortality and discharge to hospice care, particularly among patients requiring oxygen support, MV, or ECMO. Furthermore, in the absence of contraindications to anticoagulants, the prophylactic dose of heparin [49,50] is recommended.
Vaccination is recommended for older adults, and the Centers for Disease Control and Prevention (CDC) [51] currently endorses the use of the Pfizer, Moderna (Cambridge, MA, USA), and Novavax (Gaithersburg, MD, USA) vaccines. Moreover currently, the Janssen (Beerse, Belgium) vaccine is not in use. In a study [52] involving patients aged ≥70 years, both the Pfizer and AstraZeneca (Cambridge, UK) vaccines demonstrated efficacy in providing protection. Notably, among patients aged ≥80 years, both vaccines reduced the risk of hospitalization. Whereas the Pfizer vaccine was found to decrease the risk of mortality. Moreover, research [53] assessing the effects of vaccination in relation to mortality and deaths associated with COVID-19 in patients aged ≥60 years revealed that most vaccines had a favorable impact on reducing hospitalizations and deaths. Common side effects of vaccines [54] include local and systemic reactions, such as pain at the injection site, fever, fatigue, and headache. Although severe adverse events related to currently available COVID-19 vaccines are extremely rare, they have shown a minor risk of myocarditis [55,56] (more prevalent in younger individuals) and thrombosis with thrombocytopenia [57,58].
Contribute Factors of COVID-19 Severity
Older age has consistently been linked to more severe COVID-19 outcomes. According to a systematic review and meta-analysis [59], there is a correlation between age and severe outcomes of COVID-19, such as hospitalization, intensive care unit admission, MV, and mortality. Furthermore, in most studies, older age was associated with an increased likelihood of progression to severe or critical conditions [16,32,60,61] and a higher fatality rate [28,62].
Consequently, the mortality rate among older patients was notably higher than younger patients [63,64]. This finding reinforces the notion that advanced age is a significant risk factor for COVID-19 [6-9,65-67]. In addition to age, several other recognized risk factors contribute to disease severity and potentially fatal outcomes. Certain comorbidities have also been highlighted as contributing factors to COVID-19 outcomes. Conditions such as diabetes [68-70], hypertension [7,21,70], cardiovascular diseases [20,68,71], chronic kidney disease [70,72] and respiratory illnesses [7,68,73] have been consistently reported as risk multipliers for severe or fatal outcomes. Furthermore, smoking [7], obesity [68,74], and immunosuppressed states [8,74] have shown a tendency to worsen the prognosis. In addition to these comorbidities, laboratory data have provided further insight into the predictors of COVID-19 outcomes. Elevated levels of inflammatory markers such as CRP [28,75,76], D-dimer [7], intereleukin-6 [71], ferritin, and lactate [77] have been consistently associated with poor outcomes. Moreover, decreased lymphocyte counts [20,71], indicating a compromised immune response, have been observed in many more severe cases. Abnormalities in liver [63] and kidney function tests [76,78] may also be associated with poorer outcomes in COVID-19 patients. Additionally, frailty, which is often assessed using frailty scales [79] or indices [80], has emerged as another significant predictor of adverse outcomes in COVID-19 patients [81-84].
Long COVID-19 Syndrome
Long COVID-19 syndrome, often referred to as “Post-COVID conditions” or “Long COVID,” designates a range of symptoms that continue for weeks or months beyond the acute phase of a COVID-19 infection or appear after the infection has resolved (Table 1). The array of symptoms associated with long-term COVID-19 syndrome is broad, encompassing both physical and neuropsychiatric manifestations. The commonly reported symptoms are shown in Table 1 [85-94]. Management of long-term COVID-19 is currently symptomatic and supportive. Furthermore, given the diverse symptom presentations, a multidisciplinary approach is often required.
Older patients exhibit a heightened risk of persistent symptoms following COVID-19 [85] as well as the potential exacerbation of chronic conditions [95]. Notably, COVID-19 vaccinations have been suggested to reduce the incidence of long COVID-19 [86,95]. However, given that this syndrome can exacerbate a patient’s frailty, early multidisciplinary interventions [87] coupled with effective symptom management are crucial for older patients [95]. In a study [96] focused on individuals aged ≥65, patients were identified using diagnostic codes corresponding to COVID-19, influenza, and related symptoms. Outpatients with long COVID predominantly exhibited symptoms such as dyspnea, fatigue, palpitations, memory issues, cognitive impairments, sleep disturbances, and loss of taste or smell. The inpatients predominantly experienced dyspnea, fatigue, palpitations, and loss of taste or smell. Based on symptomatology, 16.6% of general patients and 29.2% of inpatients met the criteria for long COVID syndrome, consistent with findings from other studies [88].
Conclusion
Older individuals with COVID-19 exhibit distinct clinical features and face significant prognostic challenges. Therefore, a comprehensive understanding of the clinical profiles and effective management strategies is imperative for healthcare professionals to optimize care and enhance outcomes in this vulnerable population. Moreover, continued research efforts are crucial for refining treatment protocols and preventive measures tailored to older patients.
Notes
Authors’ Contributions
Conceptualization: Lee JE, Lee SI. Methodology: Lee SI. Formal analysis: Lee SI. Data curation: all authors. Validation: Lee JE, Lee SI. Investigation: Lee JE, Lee SI. Writing - original draft preparation: Lee JE, Lee SI. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.