Interstitial Lung Abnormality in Asian Population
Article information
Abstract
Interstitial lung abnormalities (ILAs) are radiologic abnormalities found incidentally on chest computed tomography (CT) that can be show a wide range of diseases, from subclinical lung fibrosis to early pulmonary fibrosis including definitive usual interstitial pneumonia. To clear up confusion about ILA, the Fleischner society published a position paper on the definition, clinical symptoms, increased mortality, radiologic progression, and management of ILAs based on several Western cohorts and articles. Recently, studies on long-term outcome, risk factors, and quantification of ILA to address the confusion have been published in Asia. The incidence of ILA was 7% to 10% for Westerners, while the prevalence of ILA was about 4% for Asians. ILA is closely related to various respiratory symptoms or increased rate of treatment-related complication in lung cancer. There is little difference between Westerners and Asians regarding the clinical importance of ILA. Although the role of quantitative CT as a screening tool for ILA requires further validation and standardized imaging protocols, using a threshold of 5% in at least one zone demonstrated 67.6% sensitivity, 93.3% specificity, and 90.5% accuracy, and a 1.8% area threshold showed 100% sensitivity and 99% specificity in South Korea. Based on the position paper released by the Fleischner society, I would like to report how much ILA occurs in the Asian population, what the prognosis is, and review what management strategies should be pursued in the future.
Introduction
Recently, many papers have been published on the clinical importance of interstitial lung abnormality (ILA), a finding incidentally found in chest computed tomography (CT) [1-5]. ILA is known to be found on chest CT in 4%–9% of smokers and 2%–7% of non-smokers [1,6-9]. When ILA is found on chest CT, if patients have symptoms of chronic cough or shortness of breath and pulmonary function tests (PFTs) show a decrease in total lung capacity or a decrease in excise capacity, additional examination or periodic follow-up tests are required because there is a high possibility of interstitial lung disease (ILD) [2-5,8].
However, since ILA is an incidental radiologic abnormality on chest CT, it can be shown various symptoms ranging from asymptomatic to respiratory symptoms at the time of discovery. Also, ILA is included in a wide range of diseases, from subclinical ILDs to early pulmonary fibrosis including usual interstitial pneumonia (UIP). So it’s been confusing for quite some time what ILA means [7,10-12]. Recently, the Fleischner society published a position paper on the definition, clinical symptoms, and management of ILA. After that, confusion about the definition of ILA has decreased significantly, and various studies are being conducted on what to do in the next step if ILA findings are found on chest CT [5,8,13,14].
The chest CT findings of ILA are ground glass opacity or reticular abnormalities, lung distortion, traction bronchiectasis or bronchiolectasis, honeycombing, and non-emphysematous cysts that are defined as at least 5% of the total lung. In particular, these lesions are considered ILA only when they are in the subpleural or central predominant in the axial plane and upperor lower-lung predominant in the craniocaudal plane (Figures 1, 2). However, a limitation is that these definitions are still determined by the subjective judgment of the reader reading the chest CT. Various future studies need to mitigate these limitations [1,3,8,13,15].
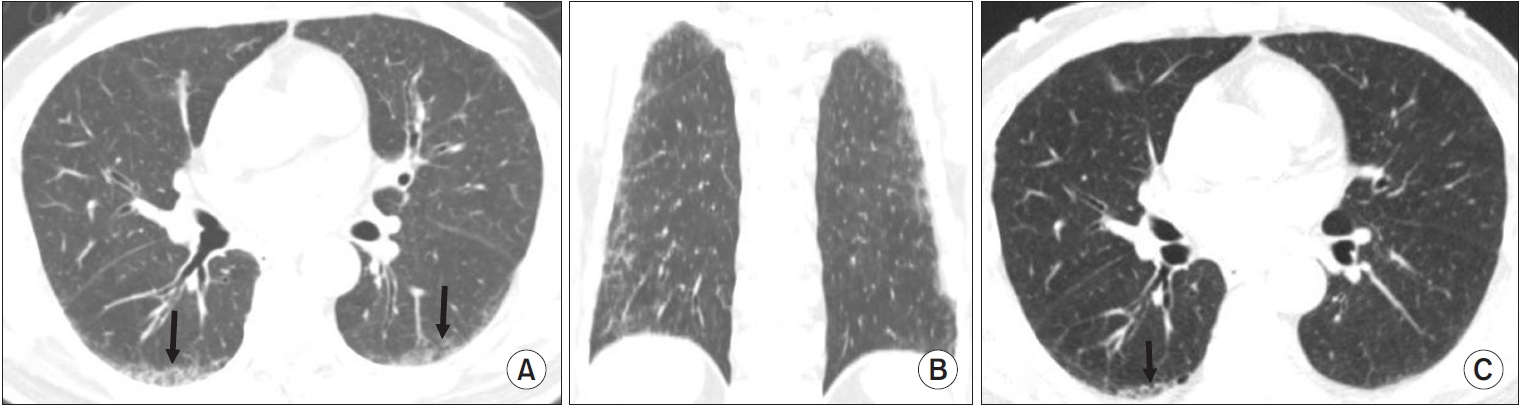
Chest computed tomography (CT) findings of interstitial lung abnormality: lower-lung predominant in the craniocaudal plane. This patient is an 81-year-old man who is heavy smoker with 40 pack-years. (A) Initial chest CT (axial plane) shows ground glass opacity with mild reticulation (arrows), traction bronchiolectasis without honeycomb in subpleural area of posterobasal segment on both lower lobes. (B) Initial chest CT (coronal plane) shows diffuse ground glass opacity with mild reticulation in subpleural area of both lungs. (C) The existing ground glass opacity with reticulation in initial chest CT improved significantly in the follow chest CT performed 9 years after smoking cessation, and it remains only in the right lower lobe (arrow).
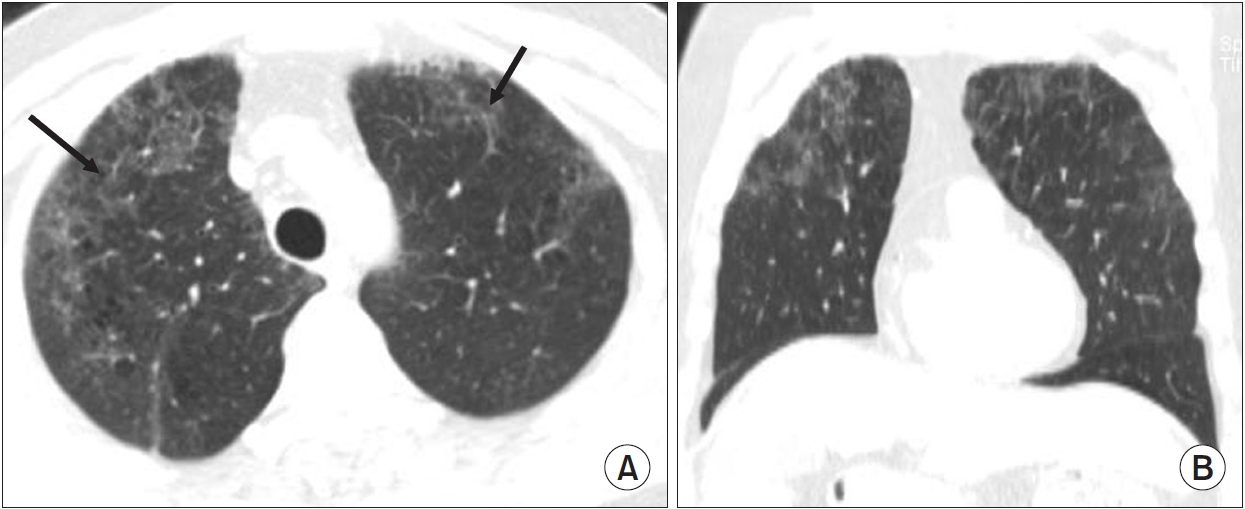
Chest computed tomography (CT) findings of interstitial lung abnormality: upper-lung predominant in the craniocaudal plane. This patient is a 63-year-old man who is heavy smoker with 40 pack-years. (A) Chest CT (axial plane) shows diffuse ground glass opacity with reticulation (arrows) in peripheral area of both upper lobes. (B) Chest CT (coronal plane) shows diffuse ground glass opacity in upper lobe predominant of both lungs.
Most research results on ILA published in the 2010s were retrospective studies on prevalence or progression in large cohorts, such as population-based cohorts or smoking and lung cancer screening cohorts, which resulted in most of the results of ILA focuses on Westerners. However, since the late 2010s, Asian countries, including South Korea or Japan, have conducted national or regional lung cancer screening low-dose chest CT for heavy smokers, and the results of research on ILA recently have been reported in these countries. In this review article, I would like to present the results of various studies on ILA in Asian countries.
Body Parts
1. Prevalence of ILA in Asians
The prevalence of ILA varied from 0.8%–22% in smokers and 2%–7% in non-smokers. The reason for the varying incidence of ILA is that the definition of ILA has been ambiguous, and there have been differences in inclusion criteria for each study [1,6-12,14,16-18]. Jin et al. [7] reported that the prevalence of ILA was about 10% of participants in the National Lung Screening Trial (NLST) (age 55 to 74 years, ≥30 pack-years of smokers). Washko et al. [11] reported that the prevalence of ILA was 10% for participants in a chronic obstructive pulmonary disease (COPD) gene study (mean age, 61 years; median smoking intensity, 36.7 pack-years). Putman et al. [18] reported that the prevalence of ILA was lower than the above reports in about 7% of participants in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study (mean age, 76 years). Meanwhile, Hoyer et al. [19] reported that the prevalence of ILA was approximately 16.7% in the Danish Lung Cancer Screening Trial (age 50 to 70 years, current or former smokers with >20 pack-years).
The prevalence of ILA differs in studies of Westerners and Asians (Table 1). In the studies reported so far, the incidence of ILA was 7%–10% for Westerners and the prevalence of ILA was about 4% for Asians, which was lower than that of Westerners [1,6,20,21]. Tsushima et al. [20] reported that interstitial changes were identified in 2.6% (80 of 3,079) of subjects (mean age 57.2±9.8 years, the mean pack-year of former and current smokers 20.7 and 29.0) who were from the rural population of the area in the Nagano Prefecture. Lee at al. [6] reported that the prevalence of ILA was 3% among patients (n=2,765) aged 50 years or older who underwent chest CT at three health screening centers. Chae at al. [1] reported that the prevalence of ILA was 4% among participates (n=3,118) in the Korean National Lung Cancer Screening Program (KNLCSP) (age 55 to 74 years, ≥30 pack-years of smokers).
It is not yet known whether the prevalence of ILA differs between Westerners and Asians. Although the reasons for the differences in prevalence are not entirely clear, the prevalence difference of ILA between Westerners and Asians may be due to a combination of genetic, environmental, and lifestyle factors. ILA is more prevalent in smokers and the elderly regardless of race, and in subjects older than 50 years who exhibit mucin 5B (MUC5B) promoter polymorphism positivity [7,13,16,18,22]. Comparing the results of the NLST published in 2013 and KNLCSP published in 2023, although the targets (age 55 to 74 years, ≥30 pack-years of smokers) were very similar, the prevalence difference of ILA was 6%, which was lower in South Koreans [1,7]. The difference in the incidence of ILA in the two groups needs to be considered from genetic factors and the definition of ILA. In the view of genetic factors, the MUC5B promoter polymorphism was associated with ILA [16]. Although it’s the result of idiopathic pulmonary fibrosis (IPF) research, the MUC5B promoter polymorphism among the non-Hispanic white population is a strong risk factor for IPF in the Mexican population, but it is very rare in the South Korean population [23]. As a result, there is a possibility that the incidence of ILA is lower in Asians than in Westerners, such as the incidence of IPF. Meanwhile, in the view of the ILA definition, a recently published ILA incidence rate of South Koreans was studied based on the definition of ILA published in 2020. Of the 3,118 participants, 120 ILAs (4%) and 95 equivalent ILAs (3%) were identified. According to the definition of ILA suggested by the Fleischner society, the prevalence of ILA was 4%, but if equivalent ILA is included, the incidence rate of ILA may be up to 7% [1]. Although the incidences of ILA and IPF are different, an interesting paper on the incidence of IPF has recently been published [24]. Maher et al. [24] reported that the authors performed a targeted literature search for population-based, observational studies reporting on the incidence and/or prevalence of IPF from January 2009 to April 2020. The adjusted IPF prevalence estimates (per 10,000 of the population) ranged from 0.57 to 4.51 in Asia-Pacific countries, 0.33 to 2.51 in Europe, and 2.40 to 2.98 in North America. In South Korea, from 2010 to 2013, the estimated IPF prevalence/10,000 was 3.97 in males and 2.43 in females. IPF incidence/10,000 was 1.64 in males and 0.97 in females. Incidence and prevalence of IPF in South Korea were similar to or higher than those in the United States and European country [25]. Since the incidence of ILA is affected by age, sex, smoking status, genetic, and environmental status, we should be constantly interested in the ILA implications of Asians.
2. Significance of ILA in Asian
The presence of ILA on chest CT is closely related to various respiratory symptoms or increased rate of treatment-related complication in lung cancer [1-4,8,13-15,21]. There is little difference between Westerners and Asians regarding the clinical importance of ILA. Respiratory symptoms are more common than in those without ILA on baseline CT (chronic cough in patients with ILA [12%] vs. chronic cough in patients without ILA [6%], p=0.006; shortness of breath in patients with ILA [18%] vs. shortness of breath in patients without ILA [9%], p=0.001) [16,20,23]. Individuals with ILA on chest CT are closely associated with reduced exercise capacity (6-minute walk distance, 219 m; 95% confidence interval [CI], 233 to 25; p=0.008) [26].
The most clinically significant result about ILA is that patients with ILA have higher mortality than patients without ILA. Various research results in healthy subjects have been published on these results, and there is a very close consistency between studies [2,7,14,18,19,27], for example, in general population studies (the Framingham Heart Study [FHS] and the AGES-Reykjavik study) or among populations of smokers assessed for COPD or lung cancer screening (Danish Lung Cancer Screening Trial) [2,8,14,16-19,23]. The hazard ratios (HRs) of increased mortality range in subjects with ILA on low-dose chest CT from 1.3 to 2.7 compared with them without ILA. Recently, studies on the relationship between the presence or absence of ILA and mortality have been published in Asian and Western countries [6,13,15]. Lee et al. [6] reported that fibrotic ILA was independently associated with disease-specific mortality (HR, 6.7; 95% CI, 3.7 to 12.2; p<0.001) and allcause mortality (HR, 2.5; 95% CI, 1.6 to 3.8; p<0.001) compared with no-ILA. Sanders et al. [27] reported that over a median (interquartile range) follow-up of 8.8 years in FHS and 12.0 years in AGES-Reykjavik cohorts, in adjusted models, ILA was significantly associated with increased mortality (HR, 1.23 to 3.08, p=0.0042 in FHS; HR, 1.41 to 1.82, p<0.0001 in AGES-Reykjavik) adjusted for multimorbidity such as cardiovascular disease (CVD), diabetes mellitus, chronic kidney disease, COPD, and cancer. In both cohorts, the association of ILA with mortality was of similar magnitude to the association of most other diseases. In adjusted models, ILA was associated only with prevalent kidney disease (odds ratio [OR], 1.90; 95% CI, 1.01 to 3.57; p=0.0452) in FHS and with prevalent CVD (OR, 1.42; 95% CI, 1.12 to 1.81; p=0.0040) in AGES-Reykjavik.
Recently, studies have been published in South Korea showing that the presence of ILA in patients with lung cancer affects treatment outcome or mortality. In the case of lung cancer accompanied by ILD, it is well known that ILD affects the prognosis of surgery, radiation therapy, and chemotherapy, but recently, it is known that the presence or absence of ILA affects treatment-related complication in lung cancer. Im et al. [28] reported that compared with the no-ILA group, the OR of the prevalence of post-operative complications of lung cancer increased to 9.56 (95% CI, 2.85 to 32.1; p<0.001) in the ILA group and the 5-year overall survival (OS) rates of no-ILA and ILA groups were 76% (95% CI, 71% to 83%) and 52% (95% CI, 37% to 74%), respectively. Patients with ILA had worse 5-year OS than those in the no-ILA group (log-rank p=0.002). Therefore, the presence or absence of ILA on chest CT before surgery is a very important finding in determining the patient’s prognosis after treatment. Also, Jeong and Kim [29] reported that in patients with resected stage IA non-small cell lung cancer, fibrotic ILA patients showed a significantly higher cause-specific mortality rate among patients with no-ILA (p<0.001). Fibrotic ILA patients had a significantly higher cause-specific mortality rate than no-ILA patients at 5-year OS rates (61.88% vs. 93.03%, p<0.001). The presence of fibrotic ILA was an independent risk factor for cause-specific death (adjusted HR, 3.22; 95% CI, 1.10 to 9.44; p=0.033). Therefore, the presence or absence of ILA on chest CT before surgery is a very important finding in determining a patient’s prognosis after treatment.
In lung cancer patients, ILA found on chest CT also has an important effect on the prognosis after chemotherapy and radiation therapy (Figure 3). Kobayashi et al. [30] reported that in patients with limited-stage small cell lung cancer, ILAs showed a higher incidence rate of radiation pneumonitis compared with no ILAs (64% vs. 10%, p<0.001). Multivariate analysis confirmed that ILAs were significantly associated with the incidence of radiation pneumonitis. In univariate analysis, patients with ILA showed poorer OS than those without ILA (median, 18.9 months vs. 67.9 months, p=0.0338). ILAs are known to pose a risk for drug-induced pneumonitis. Nakanishi et al. [31] reported that the incidence of immune checkpoint inhibitors induced ILD (ICI-ILD) was higher in patients with pre-existing ILA than that in those without pre-existing ILA. In addition, patients with ground glass attenuation (GGA) in ILA had a higher incidence of ICI-ILD than that in those without GGA in ILA. In univariate logistic analysis, ILA were a risk factor for ICI-ILD. Multivariate logistic analysis revealed that GGA in ILA was a significant risk factor for ICIILD. Therefore, clinicians should be more aware of the development of ICI-ILD in patients with ILA, especially those with GGA

Prognosis after radiation therapy in patients with lung cancer and interstitial lung abnormality on chest computed tomography (CT). This patient is a 79-year-old man who is heavy smoker with 40 pack-years. (A, B) In the initial chest CT, there is a diffuse centrilobular emphysema in both lung, and a 3 cm sized lung cancer (arrows) in the posterobasal segment of left lower lobe (LLL). In addition, there is traction bronchiectasis with reticular opacity (arrow) in right lower lobe and non-emphysematous cyst with traction bronchiectasis (arrow) in LLL. (C, D) Three months later after radiation therapy, primary lung cancer in the LLL (arrow) decreased in size, but extensive acute radiation pneumonia in the left lung (arrow) and local acute radiation pneumonia in the right middle lobe (arrow) occurred.
3. Diagnosis and quantification data of ILA in Asians
As mentioned above, the Fleischner society published a position paper on the definition, clinical signature, and management of ILA in 2020 [8]. I don’t think the ILA standards proposed by the Fleischner society need to be applied differently in Westerners and Asians. This is because the ILA standards presented by the Fleischner society were agreed to and presented by scholars from the East (n=6) and West (n=16), who are world-renowned scholars in the field of ILD, so findings of ILA that can be seen in Asians were fully reflected.
It’s already well known that ILA has been defined as purely radiological and based on the incidental identification of CT abnormalities. ILAs are non-dependent abnormalities affecting more than 5% of any lung zone (upper-, middle-, and lower-lung zones are demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein). The CT findings of ILAs included ground glass or reticular abnormalities, diffuse centrilobular nodularity, traction bronchiectasis, honeycombing, and non-emphysematous cysts. However, the Fleischner society also suggested findings that should not be judged as ILA on chest CT as follows: dependent lung atelectasis, focal paraspinal fibrosis in close contact with thoracic spine osteophytes, smoking-related centrilobular nodularity in the absence of other findings, mild focal or unilateral abnormality, interstitial edema, findings of aspiration such as patchy ground glass, and tree in bud [8,13]. As already mentioned, ILAs are non-dependent abnormalities affecting more than 5% of any lung zone. Although it is retained to exclude minimal opacities and to conform to previous published literature, the threshold extent of 5% is acknowledged to be arbitrary and subjective [1,2,4,8,15]. For this reason, it is confusing for readers to determine whether the ILA is about 5% when it is found on chest CT. In addition, when the degree of ILA found on chest CT is subjectively judged to be less than 5%, it is not yet determined whether it is not ILA or it is equivocal ILA. Therefore, the definition of ILA only when there is more than 5% of ILA on chest CT requires continuous monitoring and further research in the future.
Recently, studies have been conducted to overcome this problem through quantification of ILA (Figures 4, 5). It is being studied whether quantitative imaging of chest CT provides an objective recognition of regional disease patterns of the lung that could increase the diagnostic reliability and severity assessment of diffuse lung disease [32,33]. Especially, quantitative imaging of ILD provides reproducible quantitative measures of fibrotic parenchymal severity and fibrotic pulmonary parenchymal patterns (reticular opacity or honeycomb) that facilitate accurate diagnosis and provide an objective standard for evaluating treatment response, disease progression, and disease stratification [1,32-34]. A study in family members of individuals with familial pulmonary fibrosis showed that data-driven texture analysis could detect early interstitial changes with 84% sensitivity and 86% specificity [35,36]. Kim et al. [37] reported that the quantification system for identifying ILA using a threshold of 5% in at least one zone showed 67.6% sensitivity, 93.3% specificity, and 90.5% accuracy. Chae et al. [1] reported that deep learning-based texture analysis showed high sensitivity and specificity for detecting ILA with use of a 1.8% lung area cutoff value. The median extent of ILA calculated by the quantitative system was 5.8% for the ILA group, 0.7% for the equivocal ILA group, and 0.1% for the no-ILA group (p<0.001). A 1.8% area threshold in a lung zone for quantitative detection of ILA showed 100% sensitivity and 99% specificity. Although the role of quantitative CT as a screening tool for ILAs requires further validation and standardized imaging protocols, it may be a way to solve the ambiguous criterion of more than 5% of total lung by subjective judgment presented as the diagnostic criterion for ILA [1,32,34,37].
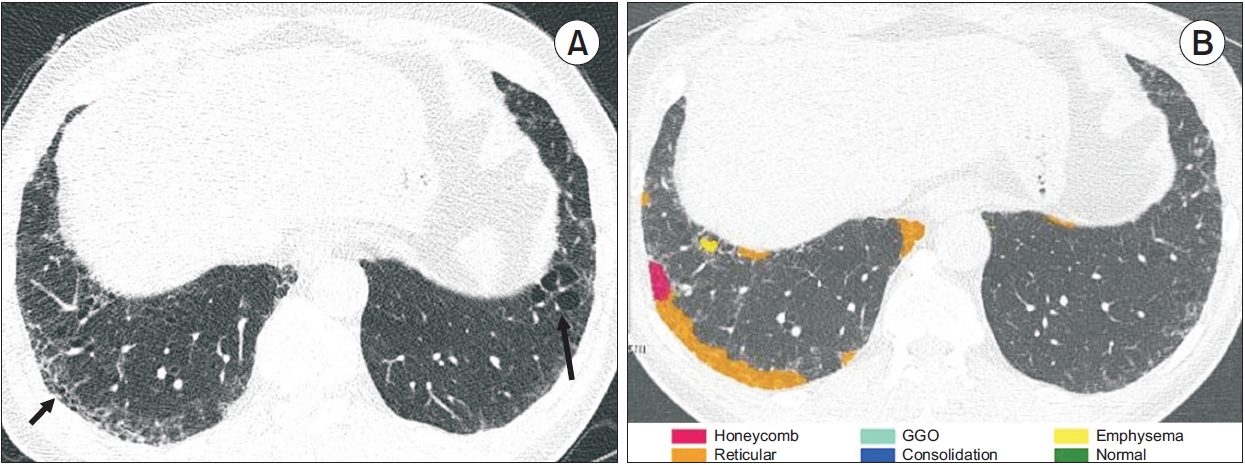
Quantification of interstitial lung abnormality on chest computed tomography (CT). This patient is a 56-yearold man who is heavy current smoker with 35 pack-years. (A) Initial national lung cancer screening low-dose chest CT showed reticular opacity with ground glass opacity (GGO) in the right lower lobe (arrow) and non-emphysematous cyst in the left lower lobe (arrow), which diagnosed interstitial lung abnormalities. (B) Five years later, follow-up chest CT scans revealed no interval change of reticulation (orange) and new focal honeycomb (red) predominantly in subpleural areas of the right lower lobes. Quantification software subclassified the images as fibrotic interstitial lung abnormality, and the mean quantitative interstitial lung abnormality extent was 1%.

Quantification of interstitial lung abnormality on chest computed tomography (CT). This patient is a 76-year-old man who is heavy ex-smoker (quit smoking 20 years ago) with 80 pack-years. (A) Initial national lung cancer screening low-dose chest CT showed reticular opacity with ground glass opacity (GGO) in the both lower lobe (arrows), which diagnosed interstitial lung abnormalities. (B) Two years later, follow-up chest CT scans revealed extension of reticulation (orange) in subpleural areas of the both lower lobes. Quantification software subclassified the images as fibrotic interstitial lung abnormality, and the mean quantitative interstitial lung abnormality extent was 2%.
4. Management of ILA
ILA is found accidentally on chest CT because most cases of them are asymptomatic. In other words, it is a chest CT finding found by accident. Therefore, in most cases, it is recommended to conduct periodic follow-up without special treatment after clinical examination [8]. However, knowing when to do follow-up, why to do it, and what the risk factors of ILA are is very important for the patient’s prognosis. Then, clinicians and radiologists need to know the schema for management of ILA so that patients can be regularly monitored when ILA is accidentally discovered on chest CT.
The most important point is that if there is potentially clinically significant ILD, ILA should be excluded. If there are no symptoms or symptoms unrelated to ILD, it is necessary to distinguish whether the ILA accidentally found on CT is definite or probable UIP. Chae et al. [15] reported that among subpleural fibrotic ILA subjects, 69% showed definite or probable UIP patterns, and 89% of subpleural non-fibrotic ILA subjects showed an indeterminate or alternative diagnosis for UIP patterns on histopathology. The CT findings for subpleural fibrotic ILAs include lung distortion, traction bronchiectasis, mild reticular opacity, and/or honeycombing. Therefore, if incidental subpleural fibrosis including honeycombing on chest CT is found, the possibility of an early ILD such a typical or probable UIP should be considered, and periodic follow-up examinations should be performed with PFTs and chest CT (Figure 6). Lee et al. [6] reported that ILA progression was observed in 80% of patients with ILA over 8 years. Subpleural fibrotic ILA was independently associated with ILA progression (HR, 10.3; 95% CI, 6.4 to 16.4; p<0.001), lung cancer development (HR, 4.4; 95% CI, 2.1 to 9.1; p<0.001), disease-specific mortality (HR, 6.7; 95% CI, 3.7 to 12.2; p<0.001), and all-cause mortality (HR, 2.5; 95% CI, 1.6 to 3.8; p<0.001) compared with no-ILA. These results have been obtained because patients with ILA have only been followed up so far, and if patients with subpleural fibrotic ILA are followed up for a long time in the future, we will have no choice but to watch them progress slowly. The CT findings of non-fibrotic ILAs include GGA and reticular abnormality (Figure 7) [1,5,6,14]. This finding is a lower risk factor for progression or mortality, and it is desirable to perform periodic follow-ups. However, close monitoring is recommended only when lung function decreases. These findings may improve during the follow-up period. Even if it worsens, it is known to take more than 10 years.

A case of interstitial lung abnormality progressing to usual interstitial lung disease. This patient is a 76-yearold man who is heavy current smoker with 40 pack-years. (A, B) Initial chest computed tomography (CT) showed reticular opacity in the right lower lobe and non-emphysematous cyst with honeycombing in the left lower lobe (arrows), which diagnosed fibrotic interstitial lung abnormalities. At this time, lung biopsy was not performed because the patient refused to take an open lung biopsy. (C) Seven years later, follow-up chest CT scans revealed extensive honeycombing in both lower lobes, which was diagnosed as typical usual interstitial pneumonia.
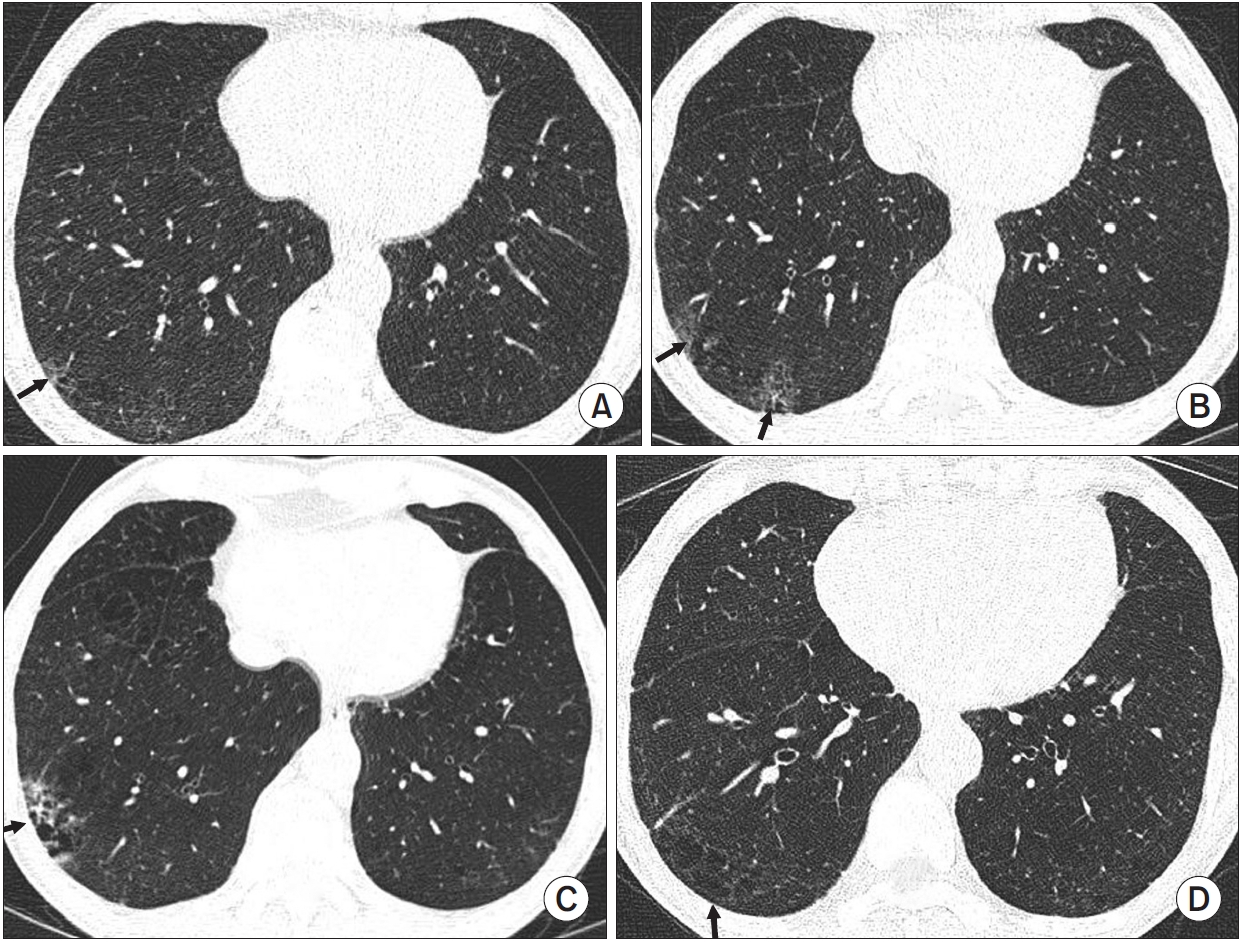
A case of progression of non-fibrotic interstitial lung abnormality. This patient is a 70-year-old man who is heavy current smoker with 30 pack-years. (A) Initial national lung cancer screening low-dose chest computed tomography (CT) showed focal reticular opacity with ground glass opacity in the right lower lobe (arrow), which was diagnosed as nonfibrotic interstitial lung abnormalities. (B) Two years later, national lung cancer screening low-dose chest CT showed progression of reticular opacity with ground glass opacity in the right lower lobe (arrow). (C) Four years later, national lung cancer screening low-dose chest CT showed more progression of reticular opacity with lung destruction in right lower lobe (arrow). At this time, open lung biopsy was performed, and pathological findings showed interstitial lung fibrosis superimposed on inflammation without usual interstitial pneumonia findings. (D) Six years later, national lung cancer screening low-dose chest CT showed a non-emphysematous cyst in the right lower lobe (arrow).
Conclusion
Recently, interest in ILA has increased in Asian, and many studies on it have been published. The prevalence of ILA was about 4% in Asians, which was lower than in Westerners. From the diagnostic criteria of the ILA, the threshold extent of 5% is still arbitrary and subjective. Although the role of quantitative CT as a screening tool for ILAs has limitations, a method of quantifying the amount of ILA using artificial intelligence may provide objective information. Finally, it is very important to know the presence or absence of subpleural fibrotic ILA in initial chest CT because subpleural fibrotic ILA, among the subtypes of ILA, is very closely related to the progression and morbidity of ILA. In addition, because subpleural fibrotic ILA is closely associated with definite or probable UIP patterns, periodic follow-up examinations should be performed with PFTs and chest CT.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.


