Tailored Biologics Selection in Severe Asthma
Article information
Abstract
The management of severe asthma presents a significant challenge in asthma treatment. Over the past few decades, remarkable progress has been made in developing new treatments for severe asthma, primarily in the form of biological agents. These advances have been made possible through a deeper understanding of the underlying pathogenesis of asthma. Most biological agents focus on targeting specific inflammatory pathways known as type 2 inflammation. However, recent developments have introduced a new agent targeting upstream alarmin signaling pathways. This opens up new possibilities, and it is anticipated that additional therapeutic agents targeting various pathways will be developed in the future. Despite this recent progress, the mainstay of asthma treatment has long been inhalers. As a result, the guidelines for the appropriate use of biological agents are not yet firmly established. In this review, we aim to emphasize the current state of biological therapy for severe asthma and provide insights into its future prospects.
Introduction
Asthma, the most prevalent chronic respiratory disease, is characterized by airway inflammation and respiratory symptoms [1]. Despite considerable progress in treatment of asthma, achievement of adequate disease control remains a challenge for some patient with asthma, resulting in uncontrolled symptoms, frequent exacerbations, and a decreased quality of life [2]. In particular, a substantial number of patients with asthma remained uncontrolled despite the optimization of asthma therapy, with administration of high-dose inhaled corticosteroids (ICS) with long-acting-beta antagonists [3]. These patients, regarded as having severe asthma, presents significant clinical challenges, highlighting the need for development of a novel therapeutic approach that can improve on conventional treatment, including inhalers and oral corticosteroids (OCS) [4].
Severe asthma is known as a heterogeneous syndrome with various proposed phenotypes [2]. Because recent studies have reported on new pathways, the conventional classification of asthma phenotypes as eosinophilic asthma and non-eosinophilic asthma has changed: type 2 high-inflammation (T2-high) asthma and type 2 low-inflammation (T2-low) asthma [5,6]. This biological heterogeneity enables targeting of specific molecules or pathways, facilitating development of biological agents for treatment of severe asthma [7]. Remarkable advancements have been achieved since the introduction of the first biological agent for treatment of asthma in 2003 [8], and rapid acceleration in the development of novel biological agents targeting different pathways has been reported [9-12]. In this review, we discuss the biologics that are currently available for use in the management of severe asthma, with an emphasis on tailored biologics selection.
Severe Asthma Phenotype
Severe asthma can be categorized according to two distinct phenotypes: T2-high and T2-low [13]. Pathways associated with T2-high asthma are the target of most biologics; therefore, this classification holds critical significance in selecting appropriate biological agents [7]. Thus, assessing the phenotype of patients with severe asthma prior to initiation of biologics is essential in order to ensure that the chosen therapy aligns with the specific inflammatory pathways observed in each patient. Figure 1 provides a concise overview of T2-high and T2-low asthma inflammation.
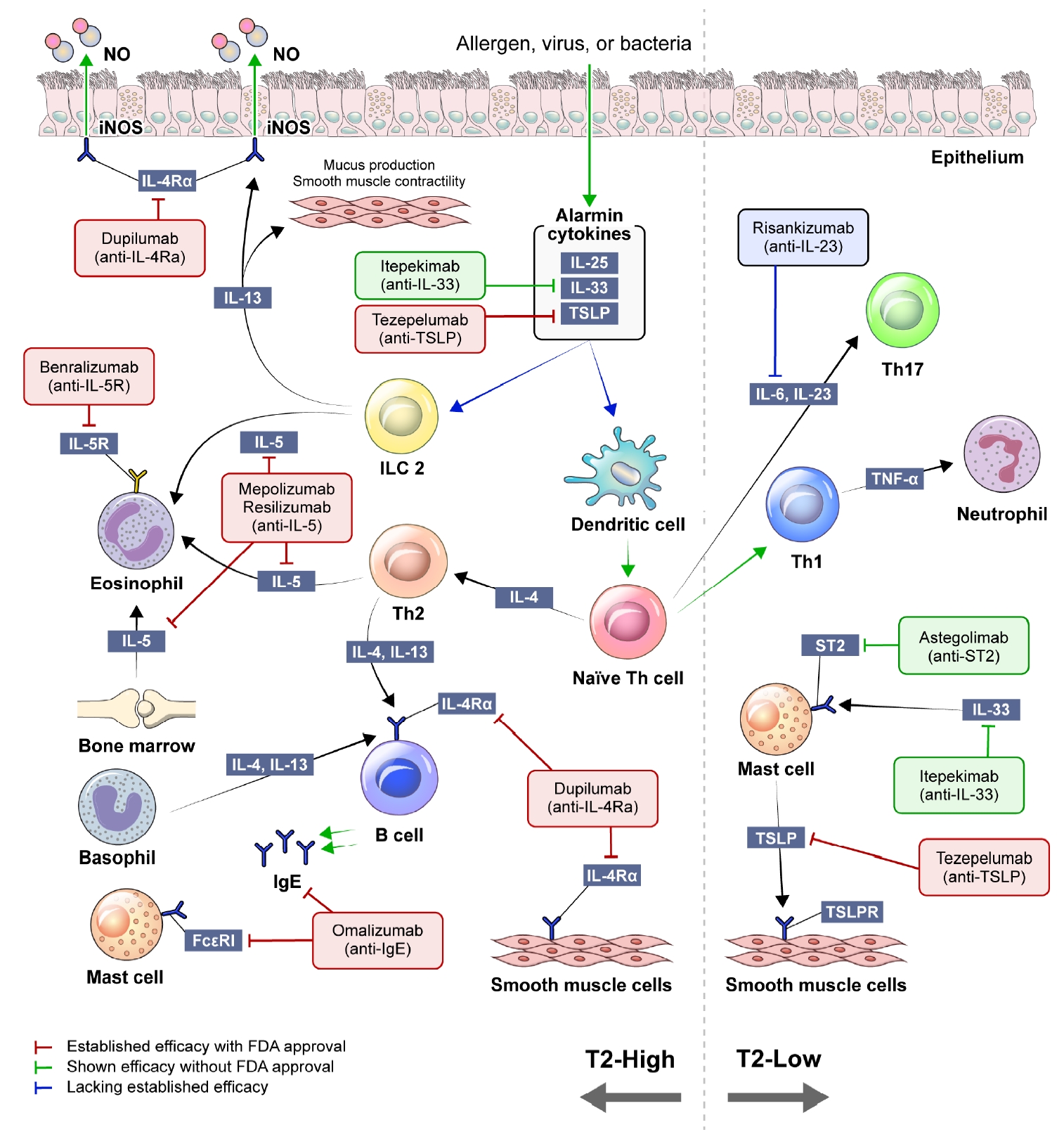
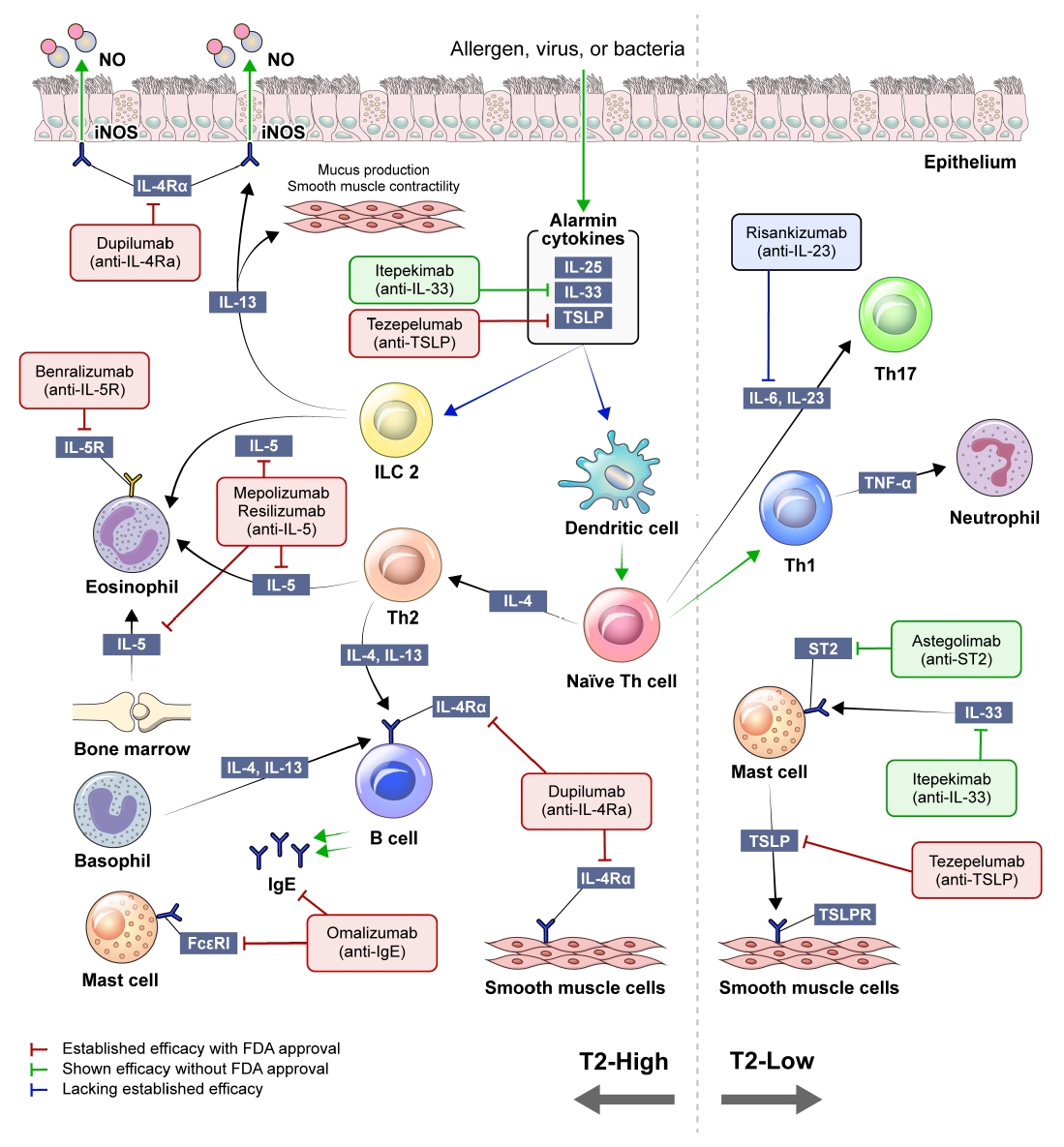
Concise schematic of type 2 high-inflammation (T2-high) and type 2 low-inflammation (T2-low) asthma inflammation. NO: nitric oxide; iNOS: inducible nitric oxide synthase; IL: interleukin; TSLP: thymic stromal lymphopoietin; ILC2: type 2 innate lymphoid cell; Th: T-helper; ST2: suppression of tumorigenicity 2; FcεRI: the high affinity IgE receptor; IgE: immunoglobulin E; TSLPR: TSLP receptor; FDA: U.S. Food and Drug Administration.
When exposed to allergens, pollutants, viruses, or bacteria, airway epithelial cells release cytokines known as alarmins, which encompass interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP). Alarmins trigger myeloid dendritic cells to promote the differentiation of T-helper (Th) 2 cells and the activation of type 2 innate lymphoid cells (ILC2s). Th2 cells and ILC2 mediate type 2 inflammation, an immune response that releases a range of cytokines, IL-4, IL-5, and IL-13 [14]. IL-4 is a key component in differentiating naïve T cells into Th2 cells and drives the switching of immunoglobulin E (IgE) isotype in B lymphocytes. The proliferation and differentiation of eosinophils in the bone marrow are promoted by IL-5. IL-13 induces the enzyme inducible nitric oxide synthase expression in airway epithelial cells, resulting in an increase of fractional exhaled nitric oxide (FeNO). It also causes mucous hypersecretion and airway smooth muscle cell contraction, leading to bronchoconstriction. Consequently, the products generated in this process, such as serum IgE, FeNO, and blood eosinophil count, have been suggested as biomarkers in type 2 inflammation [15].
T2-low asthma encompasses various phenotypes, including neutrophilic and paucigranulocytic subtypes [6]. The process related to this phenotype involves activation of Th1 and Th17 cells, along with elevated levels of IL-17A [16,17]. In T2-low asthma, onset of the disease typically occurs in adulthood, and the relatively limited efficacy of corticosteroids compared to T2-high asthma has been well established [18]. In addition, the prevalence of comorbidities, such as obesity and gastroesophageal reflux, is often higher for patients with T2-low asthma [19,20].
Available Biological Agents
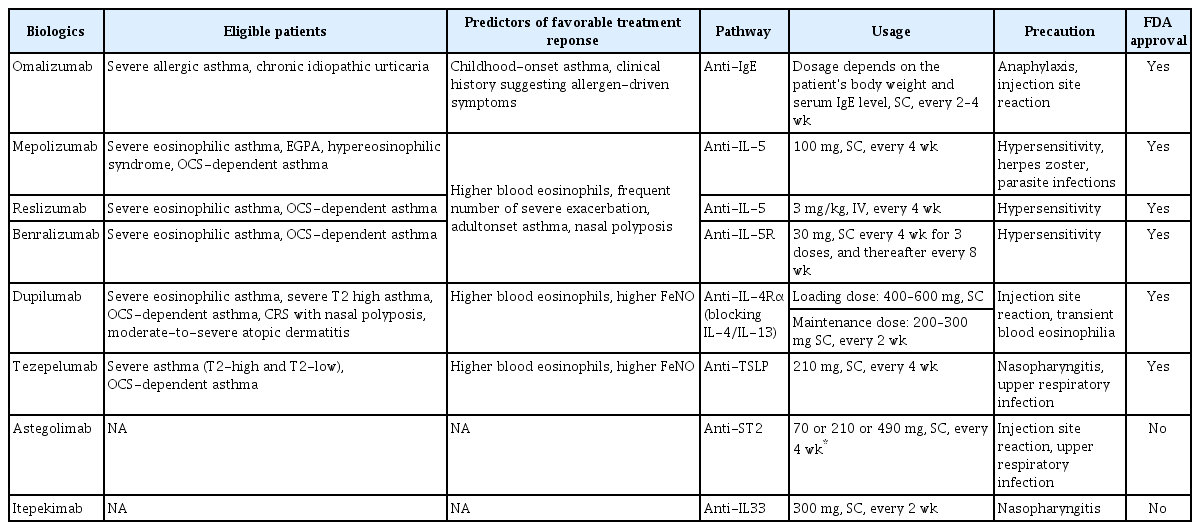
A summary of currently utilized biologics and their relevant pathways, usage, and common side effects is shown in Table 1.
1. Anti-IgE monoclonal antibody
Omalizumab is the first biological agent administered for treatment of asthma [8]. This drug functions through binding to the Fc portion of free IgE, thereby inhibiting the binding of IgE to the high-affinity IgE receptor (FcεRI) on mast cells and basophils, as well as FCεRII on dendritic cells and eosinophils. Omalizumab can be considered as an option for treatment of patients with severe allergic asthma and chronic idiopathic urticaria [21]. Its ability to reduce exacerbations in appropriately selected patients with severe asthma has been demonstrated [22]. In South Korea, patients with an increased level of serum IgE (≥76 IU/mL), sensitization to a perineal allergen, low forced expiratory volume in 1 second (<80%pred), and frequent exacerbation (≥2 times/previous year) are eligible for treatment with omalizumab. It is currently the only biological agent used for treatment of asthma that is covered by the health insurance system in South Korea. However, as mentioned above, the indication for omalizumab is narrow, and its impact on other outcomes, such as quality of life, OCS-sparing effect, and improvement in lung function, is considered modest compared to other available biological agents [23].
2. Anti-IL-5 and IL-5R antibody
IL-5 plays a significant role in differentiation and activation of eosinophils [24]. Activated eosinophils are associated with airway inflammation, resulting in the eosinophilic phenotype of severe asthma. According to the analysis of data from the National Health and Nutrition Examination Survey, 69% of adult patients with asthma had eosinophilic asthma (blood eosinophil count ≥150 cells/μL) [25]. In addition, untreated eosinophilic asthma can lead to serious complications, including contraction of airway smooth muscle, progressive airway damage, airway hyperresponsiveness, and airway remodeling [26].
Biological agents that target IL-5 include two types of drugs: mepolizumab and reslizumab, which specifically bind to IL-5, and benralizumab, an antibody that targets IL-5R [10,11,27]. The efficacy of all of these agents in reducing asthma exacerbations, as well as the role of mepolizumab in decreasing the use of oral glucocorticoid and the effect of reslizumab in improving lung function has been demonstrated [28]. While several studies have reported on the positive effect of mepolizumab in improving lung function, the results of some studies were not consistent in this regard [29]. Unlike the two previously mentioned drugs, benlizumab inhibits the proliferation and activation of eosinophils, resulting in improvement of lung function and a reduction of the OCS burden [10]. The effect of mepolizumab, reslizumab, and benralizumab was closely associated with the level of eosinophils, suggesting that patients with higher eosinophil levels are more likely to respond to these agents [30,31]. However, the association between other T2 inflammation biomarkers, such as IgE and FeNO, and anti-IL-5/IL-5R antibodies is less clear, and the predictability of IgE and FeNO levels on response to these biologics is not yet established.
3. Anti-IL-4α antibody
Dupilumab was introduced after identification of the anti-IL-5 antibody as a monoclonal antibody that binds specifically to the IL-4 receptor α subunit (IL-4Rα) [32]. The IL-4Rα plays a key role in the signaling pathways activated by IL-4 and IL-13, both of which are cytokines involved in immune and inflammatory responses. These two cytokines are closely related and share some common biological effects, largely mediated through the IL-4Rα. When IL-4 binds to its receptor complex, the IL-4 signaling pathway is activated, leading to various effects, including the promotion of Th2 cell differentiation, the induction of IgE production, and the regulation of inflammation. Similarly, IL-13 also binds to a receptor complex that includes the IL-4Rα. Upon binding, IL-13 activates signaling pathways that overlap with those of IL-4. IL-13 signaling is involved in airway inflammation, mucus production, bronchoconstriction, and airway remodeling, all of which can negatively impact lung function. Therefore, the effect of dupilumab on lung function improvement may be associated with IL-13 blockade. Its effectiveness in patients showing a high blood eosinophil count and FeNO has been demonstrated [12]. As mentioned above, use of this medication can result not only in a reduced risk of exacerbation but can also improve lung function, leading to enhanced quality of life for appropriately selected patients with severe asthma. Dupilumab can also effectively reduce and facilitate the discontinuation of chronic OCS use in patients with corticosteroid-dependent asthma [33]. In addition, it has received approval for use in treatment of chronic rhinosinusitis (CRS) with nasal polyps and atopic dermatitis [21]. Omalizumab and mepolizumab have also shown efficacy in treating CRS with nasal polyps, but dupilumab is the most effective biologic for this condition [34]. In an randomized controlled trial (RCT) that included patients with CRS with nasal polyps and comorbid asthma, treatment with dupilumab not only resulted in alleviation of nasal symptoms but also improvement of lung function and asthma symptoms [35]. These findings suggest that promising results might be obtained with use of dupilumab for management of upper and lower airway outcomes. Therefore, it can be regarded as a prioritized option for managing cases of severe asthma with this comorbidity.
4. Anti-epithelial cytokine antibody
Tezepelumab, a human monoclonal antibody, inhibits the activity of TSLP [36]. TSLP, an epithelial cytokine, plays a critical role in initiation and maintenance of airway inflammation triggered by airborne stimuli. TSLP has been implicated in promotion of neutrophilic inflammation through stimulation of dendritic cells, leading to the induction of Th17 polarization [37]. Tezepelumab can be distinguished from other biologics by its ability to target upstream epithelial cytokines rather than downstream cytokines, resulting in a cessation of the inflammatory process of asthma [38]. As a result of this distinct mechanism of action, there is potential for improvement of airway hyperresponsiveness with use of tezepelumab, a benefit that has not been observed with other medications [39]. Airway hyperresponsiveness is thought to be caused partly by the activation of mast cells within the airway smooth muscle bundle [40]. TSLP can activate these mast cells, leading to airway inflammation and hypersensitivity [41]. In a multicenter RCT involving 250 patients, the reduction in airway hyperresponsiveness to mannitol was markedly greater among those treated with tezepelumab when compared to the placebo group [42]. In another RCT, use of tezepelumab resulted in a reduction of exacerbations for asthma patients without elevation of eosinophil count (≥300 cells/μL), albeit to a lesser extent compared to those with an elevated eosinophil count [43]. Although accumulation of additional clinical experience is needed, the primary advantage of tezepelumab lies in its usability, even in the absence of a specific biomarker. Thus, it might be a promising choice for treatment of patients with severe asthma without available biomarkers, including T2-low asthma.
The effectiveness of astegolimab, a human IgG2 monoclonal antibody and a selective inhibitor of suppression of tumorigenicity 2 (ST2), IL-33 receptor, in reducing exacerbations in patients with severe asthma, irrespective of their blood eosinophil count, has been demonstrated [44]. However, despite the use of astegolimab, no improvements in lung function were observed. Thus, astegolimab may be considered as a therapeutic option for patients with T2-low severe asthma, but additional study will be required.
Itepekimab is a monoclonal antibody capable of blocking upstream alarmin IL-33. Although it has not yet been approved by the U.S. Food and Drug Administration, promising results in reducing loss of asthma control, defined by a decrease in peak expiratory flow or the need for additional use of a short-acting beta 2-agonist, while also improving lung function have been achieved with administration of itepekimab [45]. However, further confirmation will be required in order to determine its efficacy in preventing exacerbations in patients with severe asthma.
Potential Biomarkers and Biologics for T2-Low Asthma
While there are multiple therapeutic options for T2-high asthma, available options for T2-low asthma are limited [20]. As a result of their inadequate response to glucocorticoids and their ineligibility for treatment with newer targeted biologic agents, management of patients with severe asthma characterized by T2-low is very challenging [46]. Given the association between T2-low asthma and neutrophilic inflammation, biomarkers known to show a specific association with this biological process are considered potential biomarkers of interest. Several cytokines, including IL-1, IL-8, IL-17, IL-23, IL-33, interferon-γ, and tumor necrosis factor-α (TNF-α), have been proposed as potential biomarkers for severe asthma characterized by T2-low [17,47-50]. Antibodies for IL-1 (anakinra), IL-17 (brodalumab), and TNF-α (etanercept and golimumab) have been suggested as potential biological agents for this trait associated with neutrophilic inflammation [51-53]. Additionally, there was an investigation targeting Th 17 cells, which mediate neutrophilic inflammation in asthma [54]. Risankizumab, an anti-IL-23 monoclonal antibody, was under an RCT on severe asthma, but the results were unfavorable [55]. To date, the results from use of these agents in treatment of severe asthma have not been encouraging.
Selection and Switching of Biological Agents
Currently, there are no definitive criteria for initial biologic agents in the treatment of severe asthma. Therefore, phenotype and assumed endotype should be utilized in guiding the selection process, in alignment with the patient’s clinical presentation and preferences [56]. During this process, utilization of currently available biomarkers should be based on clinical evidence. These biomarkers include blood eosinophil count, FeNO, serum total IgE, and allergen-specific IgE. The current guideline recommends assessing the inflammatory phenotype when patients are on high doses of ICS [57]. Additionally, if patients are using maintenance OCS, it is advisable to discontinue or reduce the OCS dosage as much as possible before conducting the test. To firmly classify a patient as having T2-low asthma, it is recommended to perform repeated tests for blood eosinophils and fractional exhaled FeNO up to three times.
In addition, an assessment of the patient’s exacerbation history, current usage of asthma medication (including OCS), comorbidities (atopic dermatitis, CRS, and eosinophilic granulomatosis with polyangiitis), overall asthma control status, and expected cost should be performed by the clinician prior to initiation of any biological agent. Also, clinicians should consider the following factors related to the patient’s preference when choosing a treatment: the dosing interval (e.g., 2 weeks vs. 8 weeks), the method of injection (e.g., the option for self-administration), and the necessity for dosage adjustment based on body weight. Establishment of a protocol for usage of biologics based on local preferences as opposed to adopting a uniform algorithm is encouraged. An example of a protocol that could be adopted in South Korea is shown in Figure 2.
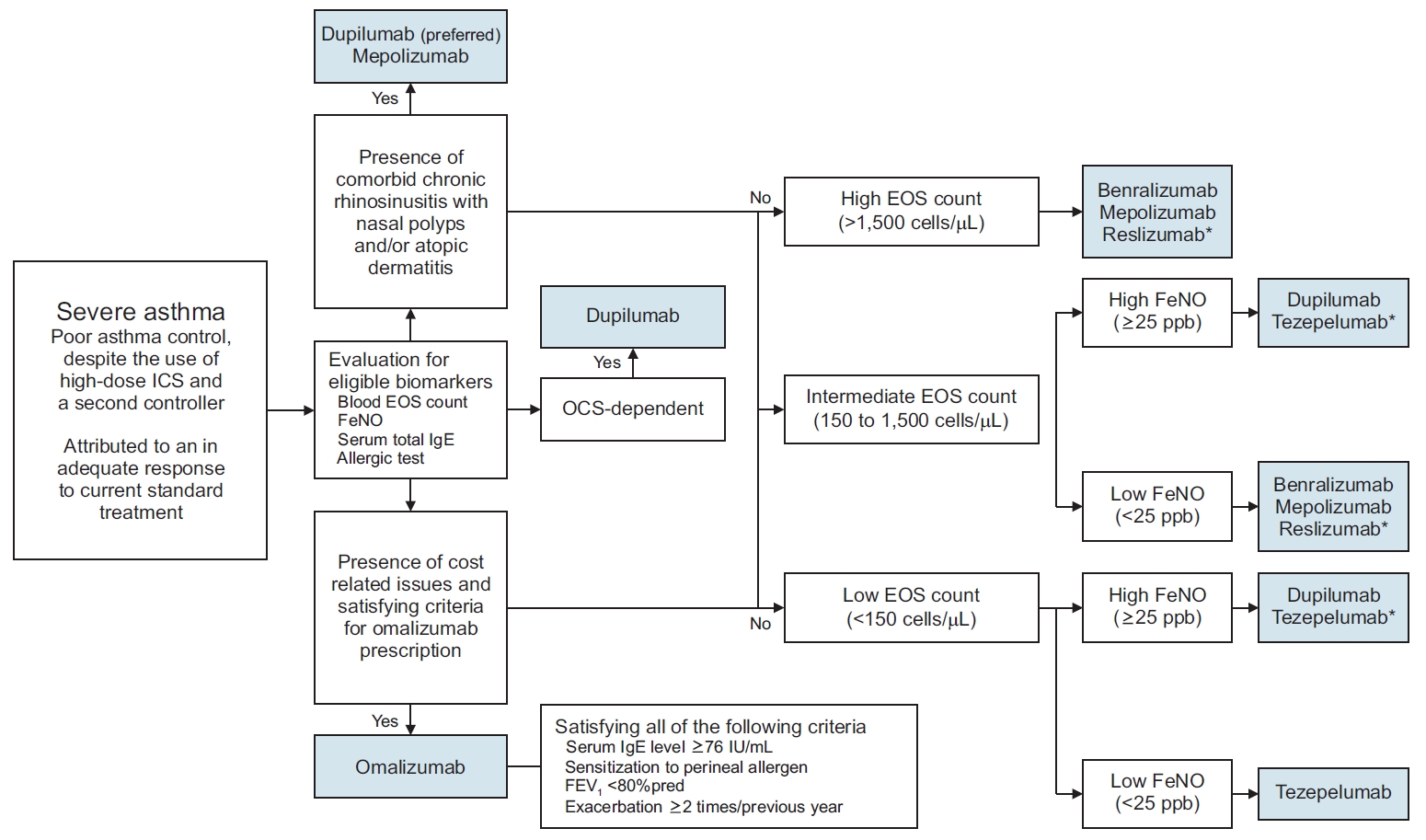
Example of a biological agent selection process. *The biologics are listed in alphabetical order. ICS: inhaled corticosteroid; EOS: eosinophil; FeNO: fractional exhaled nitric oxide; IgE: immunoglobulin E; OCS: oral corticosteroid, FEV1: forced expiratory volume in 1 second.
Despite the remarkable effectiveness of biologics in management of severe asthma, there is a subset of patients who continue to struggle with inadequate asthma control, including frequent exacerbations, even while receiving biologic therapy [58]. There are currently no clearly established criteria for evaluating the response to biologics in asthma. However, recent guideline suggests that biologics should be used for at least 4 months before evaluating the response. If the response is unclear, clinicians may consider extending the duration of treatment to 6 to 12 months. Before concluding that a patient has not responded well to a biologic, it is important to review basic factors that can be associated with a poor response to asthma treatment, such as: certainty of the asthma diagnosis, inhaler technique and adherence, and exposure to smoking or environmental factors. It is well known that patients with severe asthma are usually eligible for multiple biologics [59]. Hence, in cases where patients exhibit a poor response to their initial biologics, it is recommended to consider an alternative biological agent [60]. Before switching biologics, clinicians should conduct a reassessment of the patient’s phenotype, and it is recommended that patients discontinue their initial biologics as well as any ineffective add-on therapies. However, it is important for patients to continue using ICS.
Several studies have reported successful outcomes following the switch to a different biological agent in patients who initially showed a suboptimal response to the initial therapy. Transitioning from the anti-IgE antibody to anti-IL-5/IL-5R antibodies is the most widely investigated biologic switching strategy [27]. Results of real-world data analysis have indicated that promising outcomes can be achieved with use of this approach, including a notable reduction in exacerbations, decreased use of OCS, and improved quality of life [61,62]. In addition, successful switching from the anti-IgE antibody or anti-IL5/IL-5R antibodies to the anti-IL-4Rα antibody has been reported [63]. However, it is important to note that the number of patients enrolled in these studies is relatively small; thus, the ability to draw definite conclusions is limited. Also, patterns of biologic switching are closely related to the lead-in time of biologic agents, resulting in the most common switching being made from omalizumab [64]. To date, no well-designed large-scale prospective study that encompasses all available biological agents and examines the various possible switching scenarios has been conducted.
Conclusion
Biological agents can have a significant effect on control of asthma for patients who fail to achieve disease control despite management optimization. Previous studies have suggested that the effects of biologics can be maximized through proper consideration of phenotype and endotype. Promising outcomes have been achieved with the use of several biological agents in the treatment of severe asthma characterized by T2-high. Unfortunately, therapeutic options remain limited for T2-low asthma. However, recent study findings have raised expectations for the emergence of a novel therapy for management of T2-low asthma. However, identification of additional biomarkers and therapeutic targets for patients with severe asthma who cannot be supported with currently available biological agents is still necessary. Advancements in research will lead to increased availability of broader therapeutic options for managing patients with severe asthma in the near future.
Notes
Authors’ Contributions
Conceptualization: Kim Y. Methodology: Kim SH. Investigation: Kim Y. Writing - original draft preparation: Kim SH. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
Sang Hyuk Kim is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest.
Funding
No funding to declare.