Rigid Bronchoscopy for Post-tuberculosis Tracheobronchial Stenosis
Article information
Abstract
The healing process of tracheobronchial tuberculosis (TB) results in tracheobronchial fibrosis causing airway stenosis in 11% to 42% of patients. In Korea, where pulmonary TB is still prevalent, post-TB tracheobronchial stenosis (PTTS) is one of the main causes of benign airway stenosis causing progressive dyspnea, hypoxemia, and often life-threatening respiratory insufficiency. The development of rigid bronchoscopy replaced surgical management 30 years ago, and nowadays PTTS is mainly managed by bronchoscopic intervention in Korea. Similar to pulmonary TB, tracheobronchial TB is treated with combination of anti-TB medications. The indication of rigid bronchoscopy is more than American Thoracic Society (ATS) grade 3 dyspnea in PTTS patients. First, the narrowed airway is dilated by multiple techniques including ballooning, laser resection, and bougienation under general anesthesia. Then, most of the patients need silicone stenting to maintain the patency of dilated airway; 1.5 to 2 years after indwelling, the stent could be removed, this has shown a 70% success rate. Acute complications without mortality develop in less than 10% of patients. Subgroup analysis showed successful removal of the stent was significantly associated with male sex, young age, good baseline lung function and absence of complete one lobe collapse. In conclusion, rigid bronchoscopy could be applied to PTTS patients with acceptable efficacy and tolerable safety.
Introduction
Airway tuberculosis (TB) is a unique feature of Mycobacterium tuberculosis infection involving the tracheobronchial tree. The healing process of TB results in tracheobronchial fibrosis causing airway stenosis in 11% to 42% of patients [1,2]. In Korea, where pulmonary TB is still prevalent, airway TB is the most common cause of benign tracheobronchial stenosis [3,4]. Post-TB tracheobronchial stenosis (PTTS) may cause progressive dyspnea and often life-threatening respiratory insufficiency [5-7]. Surgical resection and reconstruction after the eradication of M. tuberculosis has been the preferred treatment option for most patients with PTTS. However, PTTS is more prevalent in young female patients, who are usually afraid of surgery. In addition, active TB infection provokes many during and after the surgical procedure complications.
The bronchoscopic intervention was developed to correct airway stenosis and to avoid surgery-related potential morbidities [8]. Balloon dilatation was initially applied; however, airway patency was maintained in less than 10% of patients, needing stenting. Metallic mesh stents were initially used to maintain airway patency but were abolished due to severe post-procedure complications, such as excessive granulation tissue formation, restenosis, stent intolerability and even stent fracture [9]. In 2005, the U.S. Food and Drug Administration (FDA) issued a warning against the use of metallic stents in benign diseases [10]. Nowadays, silicone stent is the mainstream of PTTS management [11-15]; however, it is limited by stent-related chronic complications, such as stent migration, granulation tissue formation and mucostasis. Therefore, the stent should be removed after the stabilization of the airway patency [16]. As post-inflammatory scarring and fibrosis are the main pathology of PTTS, simple ballooning and stenting could destroy the unscarred normal airway wall, because the fibrotic wall is much more resistant to circumferential dilatation [17]. Therefore, the fibrotic scar should be cut using laser, electrocautery or other mechanical modalities to avoid destruction of the normal airway wall. The narrowed airway should be widened as close to the normal as possible before stenting. Then, stenting is done to maintain the airway patency in areas affected by post-procedure fibrosis, this is aimed at achieving normal airway physiology after the stent has been removed [18-20]. This principle of airway intervention is different from vascular or intestinal stenting.
In this review, the rigid bronchoscopic treatment of PTTS will be described based on 27 years of experience.
Materials and Methods
1. Indications of rigid bronchoscopy and patents description
In PTTS patients, the indications of bronchoscopic intervention are; more than American Thoracic Society (ATS) grade 3 dyspnea, patent distal airway or less than 2 months of complete one lobe atelectasis and tolerable general condition [13,18].
Between January 2004 and December 2019, 458 patients underwent rigid bronchoscopy for PTTS [21]. Among them, 381 patients (83%) underwent silicone stenting and 77 (17%) were initially treated by ballooning or laser cauterization. Among the 344 patients with stents, 239 (70%) patients underwent successful stent removal, 96 (28%) still had indwelling stents, and nine patients (2%) underwent surgical treatment.
The median age was 43 years and female sex was predominant (82%). Rigid bronchoscopy was usually performed after the activity of TB was controlled in 91% of the patients. The most frequent symptom was dyspnea (73%) and the mean forced expiratory volume in 1 second (FEV1) was 61% of predicted.
2. Technique of rigid bronchoscopy
The bronchoscopic intervention was performed according to standard technique [11,19]. After the induction of general anesthesia, the patient was intubated with a rigid bronchoscope (Bryan Co., Woburn, MA, USA; or Karl-Storz, Tuttlingen, Germany). A flexible bronchoscope (EVIS BF 1T260, Olympus Co., Tokyo, Japan) was passed through the rigid bronchoscope, and the airway status was evaluated. Various combinations of intervention techniques have been used for rigid bronchoscopy. Stenosis was dilated by mechanical debulking and ballooning and/or using laser (neodymium-doped yttrium aluminum garnet laser [LaserSonics, Milpitas, CA, USA] or diode laser [Ceralas, Biolitec, Vienna, Germany]) therapy.
3. Selection and insertion of a silicone stent
A silicone stent (Dumon stent, Novatech, La Ciotat, France) was inserted to maintain airway patency if a pre-dilated airway showed malacia and/or circumferential inflammation. A Dumon stent with an outer diameter of 12 to 14 mm was used for tracheal stenosis, whereas a 10-mm stent was used for bronchial stenosis. According to the interventionist’s decision, an adequate size and suitable type of stent were considered.
In case of an angulated airway, an angulated stent was prepared as a silicone prosthesis cut cross-sectionally at an oblique angle using standard Mayo scissors and then reattached with 3-0 Prolene (Ethicon Inc., Cornelia, GA, USA) thread sutures to approximate the angled alignment of the tortuous airways [22]. For cases requiring Y-stents, Dumon stents with suitable length and diameter are used by making a Y-shape with Prolene sutures.
Results
1. Straight narrowing of the PTTS
Among the 381 patients who underwent silicone stenting, straight tube stent was used in most (n=336, 88%) of patients. Single lesion was the most frequent (297 patients, 86%) and the locations were the trachea in 26 patients (8%), left main bronchus in 212 (62%) patients and right main bronchus or bronchus intermedius in 41 patients (12%). Most of the patients (n=343, 90%) showed post-TB fibrotic stricture of airways, which needed mechanical widening, such as ballooning, laser cauterization, or mechanical bougienation before stent insertion. In some patients, the fibrotic lesion was thick and eccentric, which needed laser cutting instead of ballooning, because excessive ballooning could result in airway malacia by eccentric over-inflation of thin, healthy airways walls. The usual diameter of the silicone stent was 10 mm for main bronchi, 12 mm for female trachea and 14 mm for male trachea. The length of the stent was determined by adding 10 mm (5 mm for both ends) to the narrowed airway length. Multiple lesions were seen in 47 patients (14%) needing multiple stenting.
Pneumothorax developed in seven patients (2%) and respiratory difficulties were seen in six (2%), but all patients completely recovered by conservative management. No procedure-related mortality occurred. Stent-related late complications included restenosis (19%), granulation tissue formation (17%), stent migration (11%), and mucostasis (2%) (Figure 1).
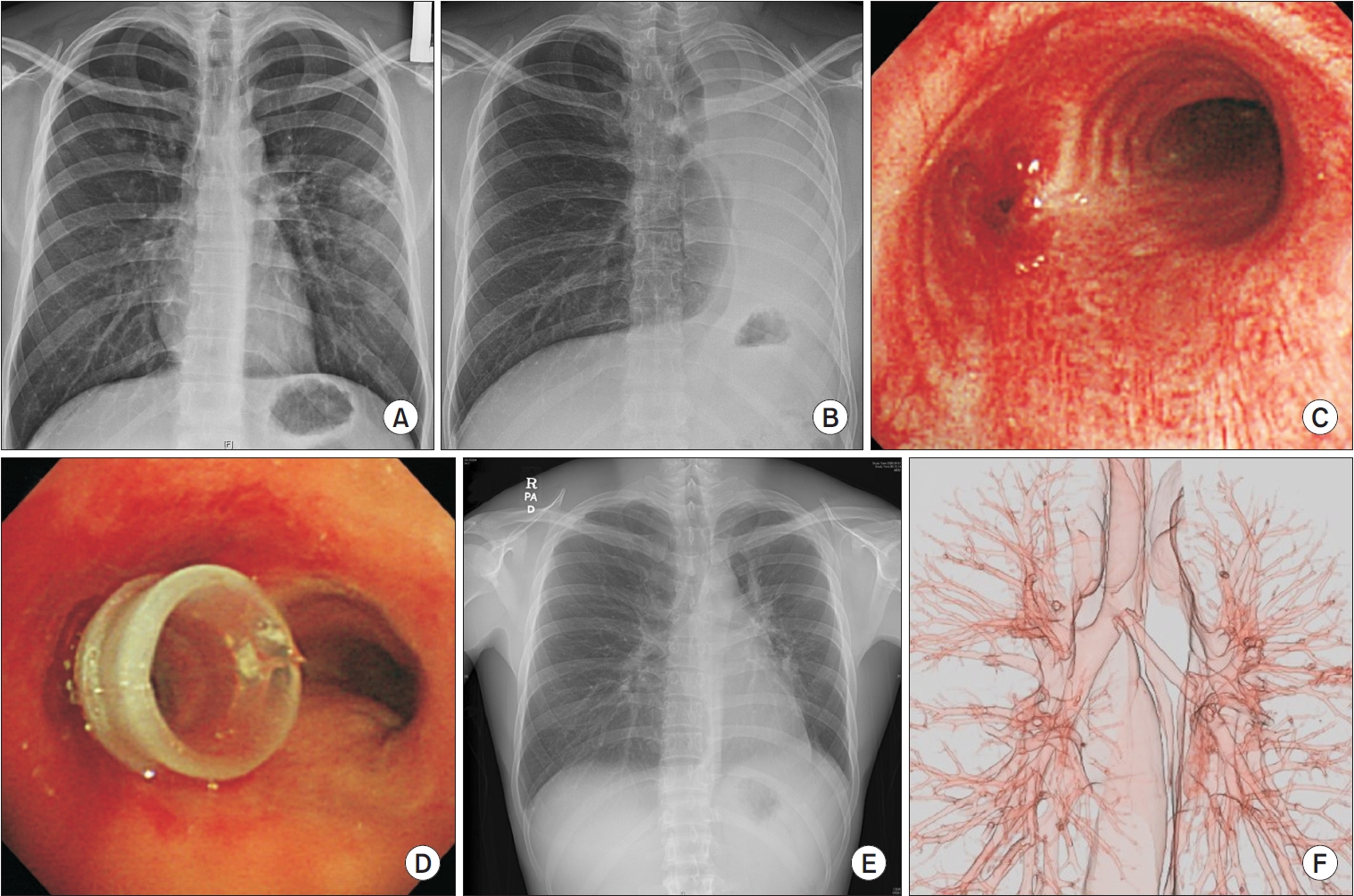
Representative pictures of post-tuberculosis left main bronchus stenosis. (A) Initial chest postero-anterior view showed ill-defined infiltration with mass forming lesion in the left lung of a 23-year-old male with positive sputum culture for Mycobacterium tuberculosis. (B) Four months after treatment with anti-tuberculosis medications, his left lung had completely collapsed. (C) Bronchoscopy showed complete stenosis of the left main bronchus by fibrosis. (D) After balloon dilatation, a 10 mm silicone stent was inserted. (E) One week after stenting, the left main bronchus became fully inflated in the chest postero-anterior (PA) view and (F) 3-dimensional computed tomography.
2. Angulated narrowing of the PTTS
Angulated airway narrowing was seen in 21 patients (6%). The representative example was left upper lobe atelectasis, which resulted in an upward curved morphology of the left main bronchus. After the straight tube stent was inserted in the angulated left main bronchus, the distal left main bronchus was frequently obstructed by the granulation tissue at the end of the stent, by the miss-alignment of the stent and bronchus. The angulated stent was made by cross-sectional cutting of straight stent at an oblique angle, and then reattaching with 3-0 Prolene sutures to approximate the angled alignment (median angulation was 140º) of the angulated airways [20]. In patients treated with angulated stents, the median duration to stent change or eventual removal was longer (392 days) than when they were indwelled with straight stents (86 days; p<0.05) [22] (Figure 2).

Representative pictures of angulated stenting in a patient with post-tuberculosis left main bronchus stenosis. (A) Initial bronchoscopy showed 75% narrowing of the left main bronchus by fibrosis in a 27-year-old female with an 8-year history of anti-tuberculosis medications. (B) After silicone stenting, her left main bronchus was widened. (C) Two months after the stenting bronchoscopy showed re-stenosis of the left main bronchus by granulation tissue. (D) After a 10 mm silicone stent was reformed in angulated shape, it was inserted into her left main bronchus. (E) One month after the angulated stenting chest postero-anterior view showed good alignment of the airway.
3. Y-stenting for multiple PTTS
Y-stent was needed in eight patients (2%). The representative example was the distal trachea and left and/or right main bronchial stenosis, which resulted in immediate migration after the straight stent was inserted. Due to the shortage of commercially supplied Y-stent, Y-stent was also made by cross-sectional cutting of straight stent and reattaching with 3-0 Prolene sutures to approximate the size and shape of the airways. All patients reported subjective symptomatic relief immediately after stent placement. No procedure-related mortality or immediate major complications occurred. Stent-related late complications included granulation tissue formation (64%) and mucostasis (18%, defined as ≥50% narrowing). The median duration of overall stent placement was 439 days (range, 119 to 1,729). The Y-stents were successfully removed in four patients (50%) after a median of 409 days [21].
4. Stent removal and long-term result
Among the 381 patients who underwent silicone stenting for PTTS, stents were removed in 285 patients (75%) after a median of 25 months. After the removal of the stent, most (n=239, 84%) of patients showed stable long-term results. Over 1 year post-removal, FEV1 was maintained at 75% of predicted. In 56 patients (20%), restenting was done due to the development of restenosis, among which, 13 patients could be stent-free later, showing a 70% stent-free rate finally [21-26].
Factors related to successful stent removal were younger age (adjusted odds ratio [aOR], 1.04; p=0.001), male sex (aOR, 5.73; p=0.011), absence of parenchymal calcification (aOR, 0.07; p<0.001), absence of segmental or lobar consolidation (aOR, 0.26; p=0.002), and more than 12 months stent indwelling duration (aOR, 6.02; p=0.013) [21,24].
Discussionand Conclusion
The overall efficacy (stent-free rate) of airway stenting was 70% in 458 PTTS patients who underwent rigid bronchoscopy over 16 years (between 2004 and 2019) [21]. The number of patients in our study is the largest pool in the world, and 16 years duration is enough to evaluate the long-term effect of stenting.
Even with effective anti-TB chemotherapy, airway stenosis develops in some patients, resulting in life-long shortness of breath and total destruction of one lobe or even one lung. In developing countries, PTTS is one of the most frequent problems in bronchologists visits; however, management guidelines are not well developed. Early diagnosis and effective chemotherapeutic treatment of TB is the key factors in the management; however, airway intervention also plays an essential role in preventing airway stenosis and loss of lung function.
Corticosteroids have been used to ameliorate airway stenosis, but a randomized control study showed no effect compared to control [27]. Surgical treatment, such as end-to-end resection and reanastomosis, was the treatment of choice before the rigid bronchoscopy became popular; however, it is less used due to the high morbidity rate of the surgery. Metallic mesh stent had for a long time been used for benign airway stenosis due to the easiness of insertion, but in 2005, U.S. FDA issued a medical device safety alert, discouraging the use of metallic stents in benign airway stenosis.
Silicone stent has many disadvantages; it is difficult to insert, needs general anesthesia and rigid bronchoscopy, and has a thick stent wall; thus, the lumen is small and easy to migrate. However, it does not penetrate the patient’s airway. Also, silicone stent could be removed or relocated as much as possible. It could be cut, sideholed, tailored for angulation and even Y-shaping. Recently 3-dimensional printing technique was used to improve central airway obstruction, but needs governmental safety approval before use.
Late complications, such as granulation tissue formation, migration and mucostasis are problematic. Mucostasis could be controlled by using the nebulizer regularly and humidifier in the dry season. Granulation tissue formation and migration could be reduced by choosing the adequate size of the stent and angulated stent in angulated airways. 3D stents could be a good solution for these problems.
In conclusion, rigid bronchoscopic intervention and silicone stenting could be used in PTTS patients with acceptable efficacy and tolerable safety.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Acknowledgements
The author acknowledge many physicians who helped to perform rigid bronchoscopy procedures, and professionals involved in patient care and article publications.
