Pancreaticothoracic Fistula Presenting with Hemoptysis and Pneumothorax in a Chronic Alcoholic Patient
Article information
Abstract
Pancreaticothoracic fistula is a rare complication of acute or chronic alcoholic pancreatitis. It may present with various symptoms, like dyspnea, abdominal pain, cough, chest pain, fever, back pain, hemoptysis, fatigue, or orthopnea. Pancreaticothoracic fistula can be detected by magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), or computed tomography. MRCP has high sensitivity and fewer side effects, and thus it has recently been recommended as the first choice for the detection of pancreaticothoracic fistula. On the other hand, ERCP enables the detection and treatment of pancreaticothoracic fistula and allows for stent insertion; for this reason it is a commonly used modality in pancreaticothoracic fistula cases. Herein, the authors describe a case of pancreaticothoracic fistula detected by ERCP and MRCP that manifested only respiratory symptoms, namely hemoptysis and pneumothorax without abdominal pain, which commonly accompanies pancreatitis.
Introduction
Pancreaticothoracic fistula is a rare complication of acute or chronic pancreatitis or pancreatic trauma, and is estimated to occur in 0.4% of patients with pancreatitis and 4.5% of pancreatic patients with a pseudocyst. The condition is most commonly related to chronic alcoholic pancreatitis.
Pancreaticothoracic fistula can present with diverse clinical findings, such as dyspnea, abdominal pain, cough, chest pain, fever, back pain, hemoptysis, fatigue, or orthopnea. Furthermore, it is difficult to confirm a diagnosis in patients without gastrointestinal symptoms in history taking.
Here, we report a case of pancreaticothoracic fistula presenting with hemoptysis, but without abdominal pain in a chronic alcoholic patient that was confirmed by endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP).
Case Report
A 56-year-old man with a history of alcohol abuse presented at an outpatient clinic with a 1-month history of progressive cough, hemoptysis, and dyspnea on exertion. He had been diagnosed with type 2 diabetes and hypertension, 2 years ago and 3 months ago, respectively. He was a heavy alcoholic, with a drinking history of two bottles of Soju daily for more than thirty years. There were no abnormal findings on physical examination. Chest X-ray on admission showed increased haziness in the right middle and right lower lobes. Laboratory evaluation at presentation revealed; a serum white blood cell count of 8.51×103/µL, hemoglobin, 8.2 g/dL, and a platelet count of 292×103/µL. Because the patient had only respiratory symptoms, other specific lab data, such as amylase or lipase levels were not checked at that time. A computed tomography (CT) scan of chest was performed to evaluate hemoptysis and findings suggestive of mediastinitis with abscess and esophageal perforation were obtained. Increased opacity and a ground glass pattern were observed in the middle and the lower lobes of the right lung (Figure 1). A sputum examination returned negative findings for acid fast bacillus (AFB) smear, sputum cytology, sputum gram staining and culture. Gastrofiberoscopy was performed to check for gastroesophageal perforation, and bronchofiberoscopy to check for the presence of an endobronchial lesion that might have caused hemoptysis, but no abnormalities were found in both. Furthermore, bronchial washing cytology showed no evidence of malignancy, and esophagography also presented no specific findings. In addition, endoscopic ultrasonography failed to depict any mediastinal lesion, and fine needle aspiration revealed only the presence of some red blood cells and inflammatory cells.
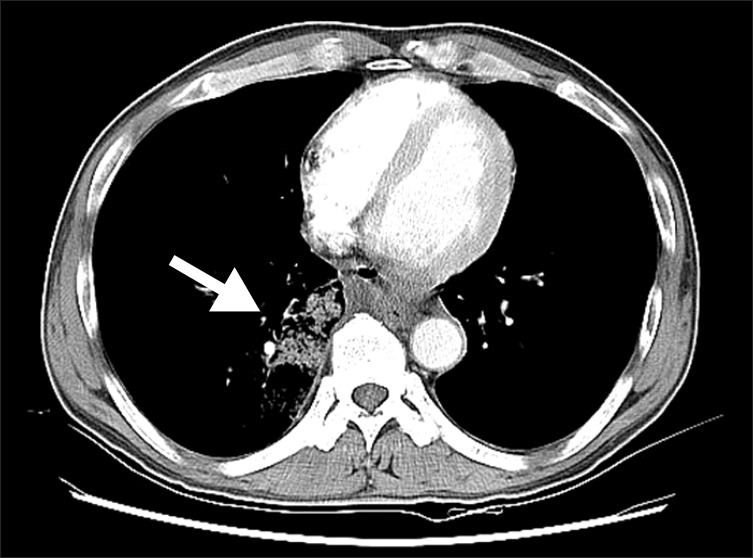
Chest computed tomography in 1st admission showing patchy ground glass opacity and consolidation in the right middle and right lower lobes caused by hemorrhage or aspirated blood, and multiple air bubbles in mediastinum suggesting esophageal perforation (arrow).
Although there was no evidence of esophageal perforation, assuming that esophageal microperforation may attribute to vessel erosion with mediastinal abscess or mediastinitis, the patient was managed conservatively with intravenous antibiotics and hemostatic agents for fifteen days, and was discharged when his symptoms resolved after 20 days of medical treatment. However, two days after discharge, cough, hemoptysis, and dyspnea re-developed, and the patient was readmitted. On second admission, vital signs were stable (blood pressure 110/70 mm Hg, heart rate 78 per minute, respiratory rate 18 per minute, temperature 36.5℃, and oxygen saturation 96% in room air). Breathing sounds were decreased but resonance was elevated on the right lung field. His sputum was foamy and brown-colored, suggestive of hemoptysis, and simple chest radiography revealed pneumothorax of the right lung. Complete blood count findings were normal, except for a hemoglobin level of 9.5 g/dL; other blood test results were C-reactive protein 1.44 mg/dL and erythrocyte sedimentation rate 62 mm/hr.
Chest CT revealed air bubbles from the lower mediastinum to the upper pancreas and a large amount of pneumothorax on the right side (Figure 2), and thus, a chest tube was inserted in the right thoracic cavity.
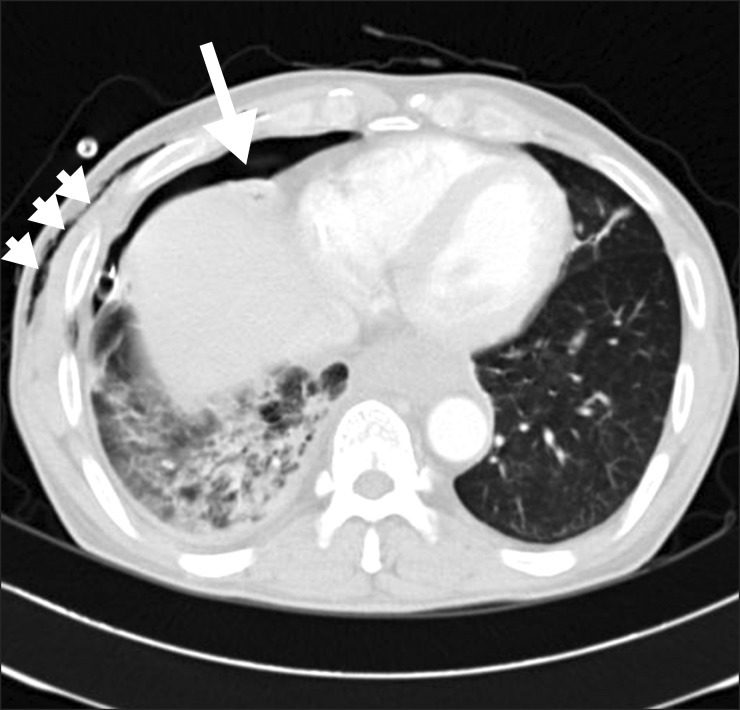
Chest computed tomography image in 2nd admission showing right pneumothorax (arrow) and subcutaneous emphysema in the right chest and abdominal wall (short-tailed arrows).
Considering chest CT findings, we assumed that there may be a pancreatic problem. Therefore, amylase and lipase levels were also checked in the serum and pleural fluid. Blood exam results were serum amylase 810 IU/L and lipase 657 IU/L. Pleural fluid analysis revealed bloody exudate with increased amylase and lipase levels (>15,000 IU/L and >3,000 IU/L, respectively) and with pleural fluid red blood cells 330,000/mm3, white blood cells 3,100/mm3 (polymorphonuclear neutrophil leukocytes 47%, lymphocyte 10%), protein 3.5 g/dL, adenosine deaminase 41.3 IU/L, and carcinoembryonic antigen 3.3 ng/mL.
Then abdominal CT was performed for further evaluation, and showed a 2.0×1.4-cm-sized air-containing cavitary lesion in the pancreatic body (Figure 3A).
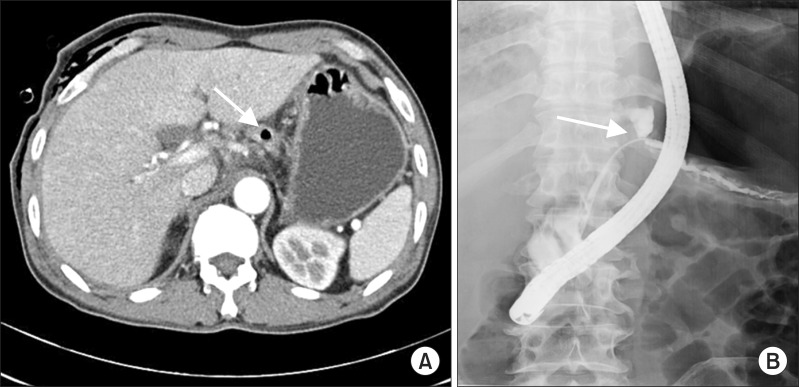
(A) Chest computed tomography image showing 2.0×1.4-cm-sized air containing cavitary lesion superior aspect of pancreatic body in peripancreatic space extending to mediastinal inflammation (arrow). (B) Main pancreatic duct disruption and dye-leakage had been discovered in endoscopic retrograde cholangiopancreatography done on the 15th day of second admission (arrow).
AFB smear and culture findings and tuberculosis polymerase chain reaction findings of pleural fluid were nonspecific.
The patient was managed conservatively with parenteral nutrition, a hemostatic agent (tranexamic acid) and intravenous antibiotics, but no improvement was observed. ERCP performed on the fifteenth day of hospitalization, demonstrated main pancreatic duct disruption with dye leakage (Figure 3B), and an endosopic nasopancreatic drainage (ENPD) tube was then placed from the nose to the pancreatic duct. On the sixteenth hospital day, the amount of hemoptysis decreased, and on the eighteenth hospital day, frothy non-bloody sputum was observed. Later, ruptured pancreatic pseudocyst and the presence of pancreaticothoracic fistula were confirmed by MRCP (Figure 4A). Two weeks after ENPD insertion, second ERCP showed that partial disruption of main pancreatic duct and pancreatic juice leakage persisted.
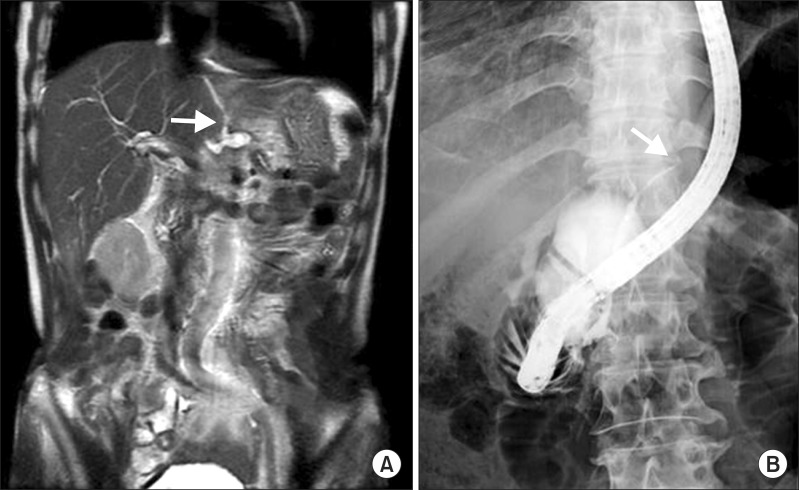
(A) Magnetic resonance cholangiopancreatography image showing a 2.3-cm-sized cavitary cystic lesion and pancreaticothoracic fistula, suggestive of pancreatic pseudocyst rupture (arrow). (B) Endoscopic retrograde cholangiopancreatography image showing resolution of the main pancreatic duct partial disruption, and a main pancreatic duct stricture, benign (arrow).
ENPD removal was then performed and an endoscopic retrograde pancreatic drainage tube was inserted on hospital day twenty-nine. At this time serum amylase and lipase levels decreased to 258 IU/L and 178 IU/L, respectively. Finally, the patient was discharged in an asymptomatic state, excepting a cough and sputum production, after 34 days of inpatient management. Follow up ERCP was performed in an outpatient clinic at 38 days after discharge, and showed resolution of the main pancreatic duct disruption, and a pancreatic body with a main pancreatic duct benign stricture (Figure 4B).
Discussion
Pancreaticothoracic fistula is a rare complication of pancreatic trauma and of acute or chronic pancreatitis, and is estimated to occur in 0.4% of patients with pancreatitis and in 4.5% of pancreatic patients with a pseudocyst1. It is most commonly related to alcoholic chronic pancreatitis2.
Pancreaticothoracic fistula can manifest as dyspnea (65%), abdominal pain (29%), cough (27%), chest pain (23%), fever (7.6%), back pain (7.6%), hemoptysis (4%), fatigue (3.8%), or orthopnea (1.9%)3.
In contrast to other pancreaticothoracic fistula cases, no chest pain or abdominal pain was present in our case. There were some case reports of pancreatithoracic fistula presenting with hemopstysis, pleural effusion, mediastinal abscess and dyspnea, but no identified case had been diagnosed with pancreaticothoracic fistula which appeared as hemoptysis and pneumothorax in South Korea. Considering that pleural effusions generally occur on the left side, although it is not rare to encounter right-sided or bilateral effusions in pancreaticothoracic fistula patients4, right lung pneumothorax by chest radiography also distinguishes this case from previously described cases.
Usually the major pancreatic duct is disrupted by pancreatitis, and this can lead to pancreaticothoracic fistula formation, and whereas anterior pancreatic ductal disruption leads to pancreatic ascites, posterior ductal leakage results in thoracic fluid collection3,5. Simple chest radiography can result in an initial diagnosis by detecting pleural effusion1,4. On the other hand, a pancreaticothoracic fistula may be suspected based on the clinical picture and much elevated pleural fluid amylase level (normal range, <150 IU/L) following thoracentesis. Moreover, although amylase elevation in pleural fluid has many possible causes, only pancreaticothoracic fistula is known to increase amylase level to beyond 50,000 IU/L1,6. In our case, the amylase level in pleural fluid exceeded 15,000 IU/L (the precise level could not be determined because the upper detection limit of the method used was 15,000 IU/L). Accordingly, other diagnostic modalities were needed.
After initial chest radiography and thoracentesis, a CT scan is often the next imaging modality to be employed in pancreaticothoracic fistula patients3.
However, the specificity of abdominal CT as a detection tool for pancreaticothoracic fistula is known to be variable, ranging from 0%7 to 100%8 in previous cases and 42% in a review of 91 patients1. In our patient, there were no definite findings suggestive of pancreaticothoracic fistula except for air bubbles found in the body of the pancreas in CT images. For the next step of the evaluation, ERCP was performed to confirm pancreaticothoracic fistula. However, although ERCP can directly demonstrate the fistulous tract1,8, its accuracy for diagnosing pancreaticothoracic fistula is also variable (from 0%9 to 100%10 in previous cases, and 78% in a review of 91 patients1). ERCP has important advantages. It enables physicians to locate pancreatic duct disruption and to plan surgical intervention of the mid or distal pancreatic duct or to drain the fistula internally with a stent placed in the pancreatic duct during ERCP, as was done in our patient. Recently, MRCP has been used to diagnose pancreaticothoracic fistula8, because it is non-invasive, does not need nephrotoxic contrast agents or sedation, has no side effects, such as, post-procedural pancreatitis, presents no infection risk, and is useful for demonstrating pancreaticothoracic fistula. Furthermore in the review of 91 patients, MRCP was found to have sensitivity of 80% for confirming pancreaticothoracic fistula, as compared with 78% for ERCP and only 47% for CT3. In the described case, MRCP was performed on hospital day eighteen, and the pancreaticothoracic fistula, which extended to mediastinum, was observed, but was not detected previously by ERCP or abdominal CT. Accordingly, we propose that MRCP could be regarded as the primary study of choice for the diagnosis of pancreaticothoracic fistula.
Pancreaticothoracic fistula can be managed medically, by stent insertion into panctreatic duct with ERCP, or surgically. However, the efficacy of medical treatment and the timing of surgical or endoscopic intervention in pancreaticothoracic fistula are controversial. A two to three-week-trial is traditionally recommended for medical treatment2, and has been reported to have an efficacy of 30-60% in terms of resolving pancreaticothoracic fistula2,3. If no improvement is achieved after two to three weeks of medical treatment, ERCP with stent insertion is recommended, but if ductal disruption and stricture are present in the head and body of the pancreas, endoscopic treatment is recommended as the first-line therapy. Early surgical intervention is considered when a stent cannot bridge the site of a ductal disruption or a downstream ductal stricture cannot be resolved by stent insertion. In cases where complete ductal obstruction exists anywhere along the main pancreatic duct, or both stricture and leakage exist within the pancreatic tail, surgical treatment should be considered as a primary management, because in such cases, the likelihood of appropriately inserting a proper stent in correct location is low. In our patient, symptoms were not resolved, after two weeks of conservative management with parenteral nutrition, a hemostatic agent, and intravenous antibiotics. Accordingly, ERCP was performed on hospital day fifteen, and main pancreatic duct disruption with dye leakage were observed. Thus, an endoscopic guided nasopancreatic drainage tube was inserted, and this patient improved in clinical symptoms, such as hemoptysis and cough, three days later.
We, herein, depict a rare case of pancreaticothoracic fistula presenting with dyspnea, hemoptysis and pneumothorax in a chronic alcoholic patient.
Many physicians tend to focus on the thoracic pathology when patients present predominantly with respiratory symptoms, and this often delays the diagnosis.
Therefore, we suggest that pancreaticothoracic fistula might be suspected when a chronic alcoholic presents with unexplained spontaneous pneumothorax and frank hemoptysis even in the absence of abdominal symptoms, or history of acute and chronic pancreatitis as was this case.
Acknowledgements
This study was supported by INHA UNIVERSITY Research Grant.
Notes
No potential conflict of interest relevant to this article was reported.