New Targeted Therapy for Non-Small Cell Lung Cancer
Article information
Abstract
Lung cancer ranks first in cancer mortality in Korea and cancer incidence in Korean men. More than half of Korean lung cancer patients undergo chemotherapy, including adjuvant therapy. Cytotoxic agents, targeted therapy, and immune checkpoint inhibitors are used in chemotherapy according to the biopsy and genetic test results. Among chemotherapy, the one that has developed rapidly is targeted therapy. The National Comprehensive Cancer Network (NCCN) guidelines have been updated recently for targeted therapy of multiple gene mutations, and targeted therapy is used not only for chemotherapy but also for adjuvant therapy. While previously targeted therapies have been developed for common genetic mutations, recently targeted therapies have been developed to overcome uncommon mutations or drug resistance that have occurred since previous targeted therapy. Therefore, this study describes recent, rapidly developing targeted therapies.
Introduction
Lung cancer is the most common cause of cancer-related deaths in Korea and the most common type of cancer among Korean men. It is ranked first in terms of age-standardized and age-specific mortality rates for both men and women. Lung cancer was the leading cause of death in Korean patients with cancer aged more than 60 years in 2021. Therefore, in terms of mortality and incidence of lung cancer, it should be considered first in Korean cancer patients [1].
Lung cancer can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) [2]. The incidence of SCLC is on the decline, but that of NSCLC continues to increase, and the most frequent histological type among NSCLC is adenocarcinoma [3,4]. At the time of diagnosis, stage 4, which represented the highest proportion among the four stages, was found in 42.0% of the patients with NSCLC.
Lung cancer treatment includes surgery, chemotherapy, and radiation therapy. Regardless of the stage of cancer, the most common initial treatment for patients with NSCLC in Korea is surgery, including adjuvant therapy (37.6%). Chemotherapy (29%) accounts for the second largest proportion of Korean NSCLC patients, and concurrent chemotherapy (4.2%) also exists [5]. It shows that the proportion of Korean cancer patients receiving chemotherapy is significantly high. Cytotoxic agents, targeted therapy, and immune checkpoint inhibitors are used in chemotherapy according to biopsy and genetic test results. Among these three, targeted therapy has developed rapidly [6]. Targeted therapy is used in both adjuvant and palliative aim. In adjuvant therapy, the National Comprehensive Cancer Network (NCCN) guidelines recommend that osimertinib can be chosen for adjuvant therapy for completely resected stage IB–IIIA NSCLC patients with epidermal growth factor receptor (EGFR) mutation [7]. According to genetic testing, target therapy is used as a first-line or second-line treatment in patients with advanced stage NSCLC. Multiple targeted therapies have been developed to overcome drug resistance and previously untargeted gene mutations. Therefore, in this article, we describe each target agent by its gene and its characteristics.
Gene Mutation Proportions in Lung Adenocarcinoma
Several types of gene mutations can lead to the development of lung cancer [8]. Figure 1 shows a chart of gene mutation proportions observed in lung adenocarcinoma for the two ethnic groups. EGFR mutations (40% to 55%) are the most common in East Asia (China, Korea, and Japan), followed by KRAS mutations (8% to 10%) and anaplastic lymphoma kinase (ALK) fusion (3% to 5%). However, in European descent (USA and Europe), KRAS mutations (20% to 30%) has the largest percentage, followed by EGFR mutations (5% to 15%), followed by ALK fusion (3% to 6%) in third place [9-12].
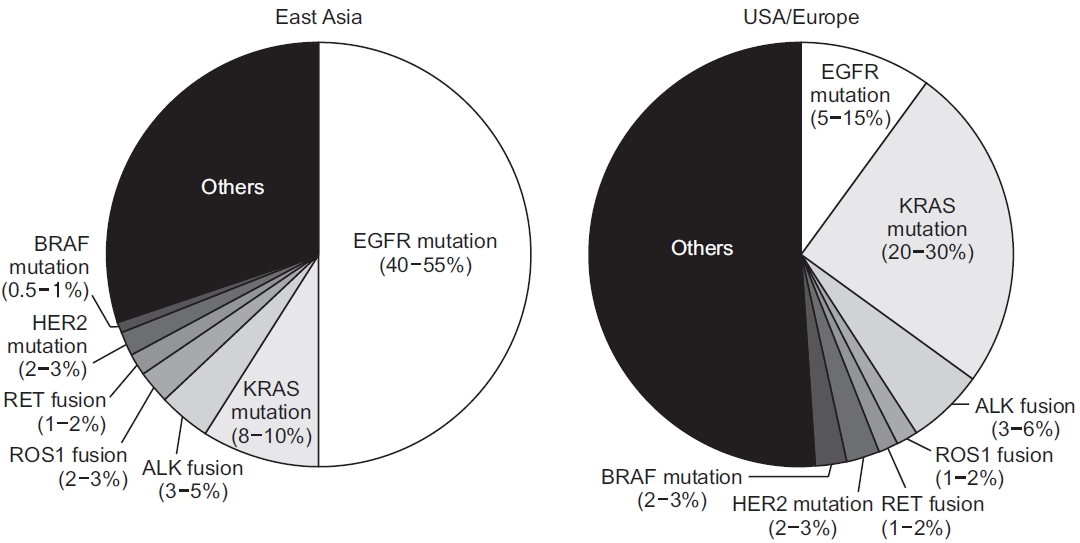
Several types of gene mutations can lead to the development of lung cancer in Europe/USA and East Asia. Adapted from Kohno et al.[9] according to Creative Commons license. EGFR: epidermal growth factor receptor; ROS1: Cros oncogene 1; RET: rearranged during transfection; HER2: human epidermal growth factor receptor 2; ALK: anaplastic lymphoma kinase.
EGFR
EGFR mutation is one of the most common mutations in all races, and EGFR-tyrosine kinase inhibitors (TKIs) are used as a standard treatment in patients with EGFR-positive advanced NSCLC.
Figure 2 shows the proportion of EGFR mutations among Caucasians and Asians [13]. The exon 19 deletion (E19 del) is the most common EGFR mutation in both Caucasians (46% to 59%) and Asians (40% to 49%). Exon 21 L858R (L858R) was the second most common EGFR mutation and exon 20 insertion (E20 INS) was the third most common mutation. The rankings were the same, but the proportions of mutations were slightly different. The proportion of E21 L858R in Caucasians (25% to 38%) was lower than that in Asians (39% to 47%). In contrast, the proportion of E20 INS in Caucasians (4% to 8%) was higher than that in Asians (2.3% to 4.5%). Other minor variations, such as Gly719Xaa (G719X), Leu861Gln (L861Q), and Ser768Ile (S768I), had similar proportions between the two races.
Gefitinib and erlotinib are first-generation EGFR-TKIs that inhibit EGFR by competitive binding with adenosine triphosphate (ATP) [14]. They showed better treatment performance than platinum-based chemotherapy, such as improved response rates and progression-free survival (PFS). However, more than 50% to 75% of gefitinib-sensitive patients showed drug resistance within 5 to 10 months, which was due to secondary EGFR gene mutations, including T790M mutations, mesenchymal-to-epithelial transition (MET) gene amplification, and overexpression of hepatocyte growth factor (HGF). Recently, the 2022 NCCN guidelines have been updated in detail for EGFR mutations, including second- and third-generation drugs. Second-generation EGFR-TKIs afatinib and dacomitinib irreversibly inhibit all four erythroblastic leukemia viral oncogene homologue (ERBB) receptors, including EGFR [15-19]. Afatinib has improved the overall response rate (ORR) and median duration of response (mDOR) compared to gefitinib and is highly effective in preventing brain metastasis. In the LUX-Lung7 (ClinicalTrials.gov Identifier: NCT01466660) clinical study, the efficacy of afatinib and gefitinib was compared in patients with brain metastasis. The PFS improvements were almost the same between the two treatments [20]. Similar results were found in a study conducted in Taiwan, but when comparing the cumulative incidence of brain metastases, it was found that afatinib had a higher brain metastases prevention than gefitinib [21]. Although the effect of afatinib is better than that of gefitinib, adverse effects such as grade ≥3 diarrhea or skin reaction are more frequent than gefitinib [22]. Patients with uncommon EGFR mutations (G719X, S768I, and L861Q) need to prioritize afatinib because it has a lower effect on gefitinib [23,24] but a higher effect on afatinib [25,26]. Dacomitinib, another second-generation EGFR-TKI, performs better than gefitinib and has longer PFS [27] and overall survival (OS) [28] results in clinical trials. In one study that saw the effectiveness of dacomitinib in patients with brain metastasis, the intracranial objective response rate (iORR) 87.5% and the intracranial disease control rate (DCR) was 100% in central nervous system (CNS) evaluable for response group [29]. Phase 2 study (ClinicalTrials. gov, Identifier: NCT04675008) is underway to find out the effectiveness of dacomitinib in advanced NSCLC patients who have not received radiation therapy. It is expected that the effect of dacomitinib on brain metastasis will be examined in more detail in the future.
After using first and second-generation EGFR-TKIs, T790M was detected in almost half of the lung adenocarcinoma patients [30,31]. To overcome this resistance, third-generation EGFR-TKIs osimertinib and lazertinib were developed. These are targeted at T790M resistance mutations generated by EGFR-TKI and are also responsive to E19 del and exon 21 L858R and L861Q point mutations. Therefore, it was necessary to consider it as a treatment option for uncommon EGFR mutations as well as T790M resistance mutations [32]. The target therapies mentioned in the following sections are third-generation EGFR-TKIs lazertinib and the newly developed amivantamab and mobocertinib for E20 INS mutations.
1. Lazertinib (Leclaza)
Lazertinib is a third-generation EGFR-TKI that target the EGFR T790M mutations. On January 18, 2021, it was first approved in Korea for patients with the EGFR T790M mutation in locally advanced or metastatic NSCLC who have previously received EGFR-TKI therapy. It also has selectivity for E19 del, L858R, E19 del/T790M, and L858R/T790M mutations [33]. It affects EGFR downstream signaling pathways and leads to cellular apoptosis. Lazertinib is more effective for brain metastatic lesions and more selective for T790M-mutant EGFR than for osimertinib in the preclinical model [34].
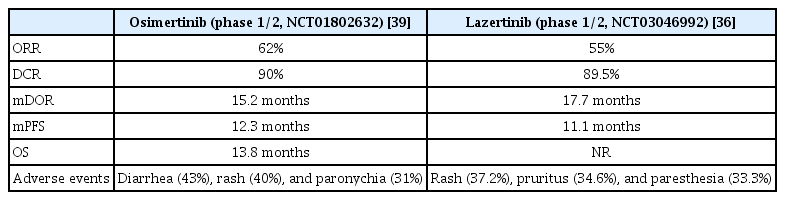
Phase 1/2 studies also showed similar results to preclinical results (ClinicalTrials.gov identifier: NCT03046992). The ORR was 55% when lazertinib was used in T790M-positive patients with advanced cancer after being treated with first- and second-generation EGFR-TKIs. The duration of response (DOR) was 17.7 months and PFS was 11.1 months. These results are similar to those of osimertinib (Table 1). Among patients with intracranial metastasis (n=7), including asymptomatic or stable brain metastases, one (14.3%) had a complete response (CR) [35] and five (71.4%) had a partial response (PR). The iORR was 86% and the PFS was 26.0 months, which was an encouraging result [36]. Common side effects were rash (37.2%), pruritus (34.6%), and paresthesia (33.3%). Serious drug-related adverse events included gastritis, pneumonia, and pneumonitis.
The most common resistance mechanism to lazertinib is EGFR T790M loss, which is similar to that to osimertinib [37]. Other resistance mechanisms involve receptor tyrosine kinase amplification and the mitogen-activated protein kinase (MAPK)-phosphoinositide 3-kinase (PI3K) pathway. Cardiotoxicity has been reported to be due to corrected QT interval (QTc) prolongation or decreased left ventricular ejection fraction (LVEF) with osimertinib. Depending on the severity of the cardiotoxicity, osimertinib treatment can be suspended or discontinued. Osimertinib may have caused cardiac repolarization by inhibiting the human ether-ago-go-related gene (hERG) potassium channels, resulting in QTc prolongation.
Inhibition of ErbB2 or human epidermal growth factor receptor 2 (HER2) and the AMP-activated protein kinase (AMPK) pathway may decrease LVEF. However, lazertinib is more selective than osimertinib, and the cardiotoxicity of lazertinib was predicted to be lower than of osimertinib. This can be confirmed in the lazertinib phase 1/2 study (Clinicaltrials.gov identifier: NCT03046992). Measurements of QTc and LVEF in study patients showed that none of the patients had QTc above 500 ms or increased for more than 60 ms, and no one had LVEF below 50% and decreased LVEF more than 10% at the same time [38]. However, lazertinib also has disadvantages compared to osimertinib for a high rate (33.3%) of paresthesia (Table 1) [36,39]. Phase 3 clinical trials (LASER301, ClinicalTrials.gov Identifier: NCT04248829) as for first-line treatment in patients with EGFR-mutation-positive locally advanced or metastatic NSCLC are in progress, and it compares lazertinib and gefitinib.
Several other studies have been conducted on lazertinib. First, MARIPOSA (Clinicaltrials.gov identifier: NCT04487080) is a clinical experiment confirming the effectiveness of lazertinib alone or in combination with amivantamab compared to osmertinib [40]. Second, another study (LU21-16, ClinicalTrials.gov Identifier: NCT05277701) was conducted to determine the efficacy of lazertinib in patients with NSCLC harboring uncommon EGFR mutations. Third, The Safety and Efficacy study (ClinicalTrials.gov Identifier: NCT05167851) of first-line lazertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR-mutant NSCLC is underway.
2. Amivantamab (Rybrevant)
E20 INS mutations are the third most common type of EGFR mutations in NSCLC. They are characterized by in-frame insertions and duplications near the C-helix of the EGFR kinase domain [41]. This change is structurally similar to that in activated wild-type EGFR. Consequently, first- and second-generation EGFR-TKIs have limited effects on E20 INS mutations [42-45]. In line-unspecified settings, PFS is 1 to 3 months [46]. Amivantamab is the first EGFR-MET bispecific antibody to bind to the receptor’s extracellular domain and detour TKI binding site resistance in patients with EGFR E20 INS, MET amplification, and C797S and T790M mutations [47].
In the phase 1 study (CHRYSALIS), amivantamab was administered to the efficacy group (n=81), which received previous platinum-based chemotherapy with an EGFR E20 INS mutation (ClinicalTrials.gov Identifier: NCT02609776). ORR of the group was 40%. Four percent of patients showed a CR and 36% achieved PR. PFS and OS are 8.3 and 22.8 months, respectively. Grade ≥3 adverse events including hypokalemia, diarrhea, rash, pulmonary embolism, and neutropenia were observed in 35% patients [48]. There are other studies besides the effects on E20 INS. FLAURA (ClinicalTrials. gov Identifier: NCT02296125) and AURA3 (ClinicalTrials.gov identifier: NCT02151981) are clinical trials of third-generation osimertinib. These studies reported that the biggest cause of osimertinib resistance is MET amplification [49,50]. Phase 3 clinical trials (MARIPOSA, Clinicaltrials.gov identifier: NCT04487080) examining the effectiveness of amivantamab plus lazertinib in treatment-naive NSCLC with EGFR E19 del or L858R mutation are currently underway.
3. Mobocertinib (Exkivity)
Like amivantamab, mobocertinib is a drug developed to target EGFR E20 INS mutations. While the amivantamab administration route is an intravenous infusion, mobocertinib has been developed to be administered orally. Mobocertinib covalently interacts with cysteine 797 in EGFR. This particular bond contributes to the selective and lasting effects of mobocertinib, which are also found in afatinib and osimertinib. However, mobocertinib has a higher affinity for EGFR E20 INS mutants than osimertinib because of mobocertinib’s isopropyl ester. This is because isopropyl ester allows the structural differences between wild-type EGFR and EGFR E20 INS mutants to be distinguished [51].
A phase 1/2 open-label nonrandomized clinical trial studied (ClinicalTrials.gov identifier: NCT02716116) mobocertinib in platinum-pretreated patients with EGFR E20 INS mutations (Table 2). The ORR was 28% based on the independent review committee assessment. The DOR was 17.5 months. PFS was 7.3 months, and OS was 24 months.
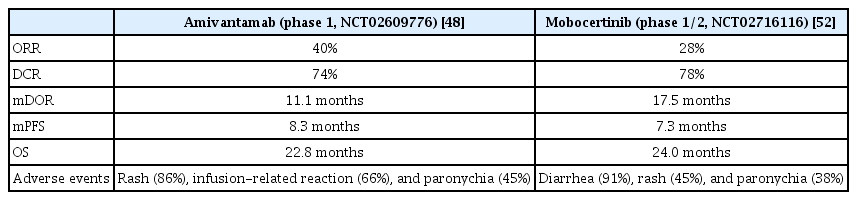
Comparison of EGFR-tyrosine kinase inhibitors for exon 20 insertion mutations in platinum-pretreated patients with EGFR exon 20 insertion mutations
The most common side effect of grade 3 or higher was diarrhea (21%), followed by stomatitis, increased lipase, and prolonged QT [52]. The Food and Drug Administration (FDA) approved mobocertinib for patients who received prior platinum-based chemotherapy with locally advanced or metastatic NSCLC with EGFR E20 INS mutations.
ALK
ALK is the third most frequent mutation in lung adenocarcinoma, with a rate of approximately 5%, measured as ALK-positive, without significant differences in race, mainly among women and non-smokers (Figure 1). The first-generation ATP-compatible ALK inhibitor is crizotinib. It was created after ALK rearrangement is known to be a potential oncogenic driver that causes NSCLC. Crizotinib also showed drug resistance after a certain period of time; therefore, the second-generation drugs, ceritinib, alectinib, and brigatinib, appeared later. Recently, a third-generation drug, lorlatinib, has been developed and approved by FDA [53].
1. Lorlatinib (Lorbrena, Lorviqua)
Lorlatinib is a third-generation ALK inhibitor targeting both ALK and C-ros oncogene 1 (ROS1). While crizotinib shows poor CNS penetration, lorlatinib is effective for brain metastasis as it can pass through the blood-brain barrier. Preclinical studies have shown that lorlatinib is effective for resistance mutations occurring in crizotinib [54,55].
A phase 2 study (ClinicalTrials.gov Identifier: NCT01970865) was conducted on 30 TKI-naive patients who were ALK-positive and 59 patients who previously received crizotinib with ALK positivity. There was an objective response of 90% in only TKI-naive patients, but patients who previously received crizotinib had an ORR of 69.5%, which was smaller than that in TKI-naive patients. The DCR showed a similar tendency to the ORR (Table 3). In contrast, intracranial responses were confirmed in 66.7% of TKI-naive patients, and in 87% of patients who had previously received crizotinib. In the previously received crizotinib patient group, the intracranial response was higher than that in the TKI-naive group; however, it may not be clear to compare the rates because the absolute number of TKI-naive patients including brain metastasis was low (two out of three patients with brain metastasis showed ORR) [56]. In another study, when fewer treatment lines remained, the ORR, DCR, and iORR values were lower than that reported in the previous study [57].

Results of clinical trials using lorlatinib in TKI-naive patients and patients who previously received crizotinib and one or more prior second-generation ALK-TKIs
For a definite comparison between lorlatinib and crizotinib, a phase 3 study (CROWN, ClinicalTrials.gov Identifier: NCT03052608) was conducted to compare whether lorlatinib was superior to crizotinib when used as first-line treatment in ALK-positive NSCLC. Intermediate results showed longer PFS and higher intracranial response when lorlatinib was used in the TKI-naive group; however, the disadvantage was the presence of more grade 3 or higher adverse event [53,58,59]. Despite the increased efficacy of lorlatinib compared to that of crizotinib, there are arguments as to whether lorlatinib should be used as first-line treatment. First, the quality of life is poor owing to multiple adverse events that occur at a high rate when loratinib is used. Lorlatinib had high rates of adverse events, such as hypercholesterolemia, hypertriglyceridemia, edema, and grade 3 weight gain, in the CROWN study. In addition, 21% of the patients had cognitive adverse effects and 16% had mood-related adverse effects. Second, the use of previous-generation drugs results in a long PFS. Therefore, there is no clear basis for using lorlatinib as first-line treatment at the expense of quality of life, and the actual benefits of using lorlatinib will be clearly calculated once the CROWN study is completed to prove the superiority of lorlatinib [60].
KRAS
KRAS mutations are common in lung adenocarcinoma. In Europe, KRAS mutations are the most frequent genetic mutations (20% to 30%), followed by those in East Asia (8% to 10%) (Figure 1). KRAS G12C mutation is the most common KRAS mutation. Previous study showed 9.8% of Chinese NSCLC patients had KRAS mutations and 29.5% of KRAS mutations appeared to have KRAS G12 mutations [61].
KRAS encodes guanosine triphosphate (GTPase), which act as a molecular switch and circulates in active GTP-bound and inactive guanosine diphosphate (GDP)-bound states. When the KRAS G12C mutation occurs, an active form of KRAS is preferred, resulting in abnormally high concentrations of GTP-bound KRAS and overactivation of uncontrolled cell growth. There have been many attempts to develop KRAS inhibitors, but it has been difficult to develop target materials because of complex molecular biological activity mechanisms and small binding sites. Sotorasib was the first approved drug to be introduced.
1. Sotorasib (Lumakras)
Sotorasib is a KRAS inhibitor that irreversibly binds to switch II regions, which exist only in the inactive GDP-bound configuration. Owing to this combination, the inactive state continues, resulting in KRAS oncogenic signaling interference.
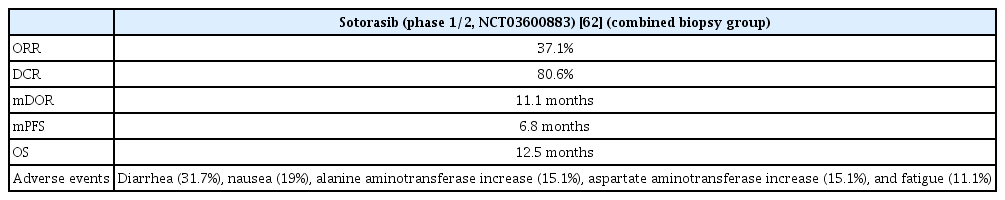
In the phase 1/2 study (CodeBreaK 100, ClinicalTrials.gov Identifier: NCT03600883), the use of sotorasib resulted in an objective response of 37.1% and disease control of 80.6%, with a CR of 3.2% and PR of 33.9%. In 46 patients with an objective response, the mDOR was 11.1 months, median PFS was 6.8 months and median OS was 12.5 months. Diarrhea was the most common adverse event, and an increase aspartate transaminase (AST)/alanine transferase (ALT) ratio was also observed (Table 4). The AST/ALT increase was the most common in grade 3 or higher adverse events. A phase 3 study (CodeBreaK200, ClinicalTrials.gov identifier: NCT04303780) is in progress to compare sotorasib and docetaxel in previously treated advanced NSCLC patients with KRAS G12C mutation [62].
MET
MET is a proto-oncogene that encrypts the transmembrane receptor tyrosine kinase, which is activated by the stromal ligand HGF. Signals mediated by HGF promote biological activities, such as cell proliferation and angiogenesis, uncontrolled cell proliferation, and various types of cancer. It can occur if the signal is not regulated because of abnormal activation of MET, such as MET amplification or point mutations [63]. Among them, one of the common mutations of MET is MET exon 14 skipping (METex14). It has an overall frequency [64,65] of 2.7% to 3% considering the total age and histological subtype in NSCLC, and the disease rate is higher over the age of 70. Although its prevalence is not high, there is a need to develop therapeutic drugs due to poor prognosis. METex14 encrypts the MET receptor intracellular juxtamembrane domain and regulates receiver tyrosine kinase activity. The occurrence of METex14 alternations at RNA slice acceptor or donor sites increases the stability and carcinogenicity of MET because the MET juxtamembrane domain disappears, ubiquitination is damaged, and the turnover of MET is reduced, eventually strengthening the signal [66-68]. Therefore, the need for a target agent specific to METex14 is required, and the recently approved drugs are capmatinib and tepotinib by the FDA.
1. Capmatinib (Tabrecta)
Capmatinib is a selective MET inhibitor. It has been approved by the U.S. FDA for NSCLC patients whose tumors have a mutation leading to METex14 [69]. In METex14 NSCLC, there is a high probability of bone metastasis and brain metastasis. In a retrospective review of 148 NSCLC patients with METex14, bone metastasis was 49% and brain metastasis was 37%. Capmatinib is expected to be useful in treating patients with METex14 NSCLC, as it can pass through the blood-brain barrier [70,71]. The actual effect of the drug was confirmed in a phase 2 study (Geometry Mono-1, ClinicalTrials.gov Identifier: NCT02414139) (Table 5). Treatment-naive METex14 patients had an ORR of 68% and DOR of 12.6 months. In the patient group using capmatinib above the second-line, the ORR was 41% and the DOR was 9.7 months. This difference was due to the possibility that patients treated previously may have a longer history of the disease, and resistance may have occurred since the previous treatment. It was also effective for brain metastasis, with 92%, 31%, and 23% showing intracranial disease control, CR, and PR, respectively.
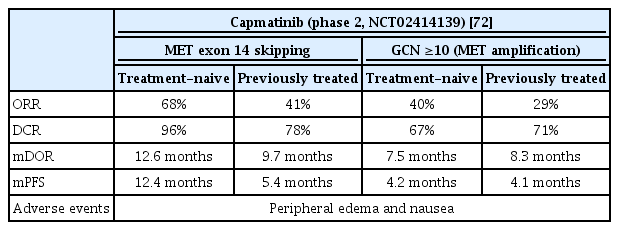
Clinical trial results for capmatinib in patients with MET exon 14 skipping mutations and MET amplification
Geometry Mono-1 study also investigated the effect of capmatinib on MET amplification; when the gain copy number (GCN) was more than 10, the ORR in the first-line therapy was 40%, and the median DOR 7.5 months. The ORR above the second-line was 29%, and the median DOR was 8.3 months. However, MET amplification in NSCLC patients with fewer than 10 GCNs showed limited activity. In conclusion, capmatinib is effective in NSCLC patients with a GCN more than 10 times METex14 or MET amplification, based from a phase 2 study. The most common adverse events was peripheral edema, followed by nausea. Peripheral edema was also the most frequently generated adverse effect of grade 3 or higher, followed by dyspnea, increased ALT, pneumonia [72,73].
2. Tepotinib (Tepmetko)
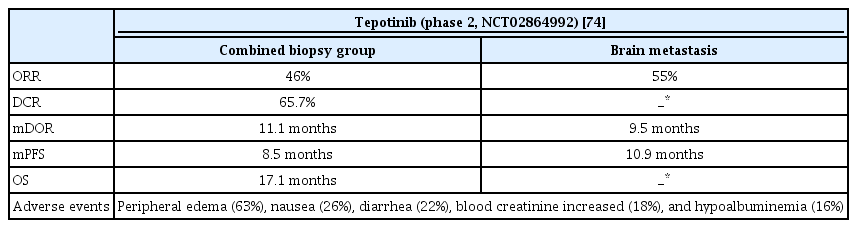
Tepotinib is a highly selective oral MET inhibitor approved by the FDA after capmatinib. In a phase 2 study (VISION, ClinicalTrials.gov Identifier: NCT02864992), similar results were obtained for tepotinib (Table 6). Based on an independent review, ORR of 46% and mDOR of 11.1 months were found, and ORR was similar regardless of previous treatment. For brain metastasis, ORR was 55% based on an independent review, mDOR was 9.5 months, and PFS was 10.9 months. The most common adverse event was peripheral edema (63%), followed by nausea and diarrhea. In cases of grade 3 or higher, peripheral edema was the most common, followed by pleural effusion, and amylase/lipase levels increased [74].
MET amplification is one of the mechanisms by which acquired resistance to EGFR-TKIs occurs in patients with NSCLC using osimertinib as the first-line treatment. This ratio is approximately 7% to 15% of the total mechanism (Figure 3) [37]. As capmatinib can also act on MET amplification mutations, clinical studies (INSIGHT 2, clinicaltrials.gov, Identifier: NCT03940703) using tepotinib and osimertinib together in osimertinib relapsed MET amplified NSCLC patients are underway.
RET
Rearranged during transfection (RET) encodes a transmembrane receptor tyrosine kinase related to normal embryonic development of the nervous system and kidneys. If the 5’ sequences of another gene are juxtaposed with the RET 3’ array that encrypts the intracellular tyrosine kinase domain through inversion or translation of the chromosome, RET fusion occurs. RET fusion is an oncogenic driver that occurs in 1% to 2% of NSCLC patients and has a high rate in young women, non-smokers, and lung adenocarcinoma [75,76]. In patients with RET fusion-positive NSCLC, the need to develop gene-specific target agents has emerged because of the high risk of brain metastasis and poor prognosis. Accordingly, RET inhibitors selpercatinib and pralsetinib were developed.
1. Selpercatinib (Retevmo)
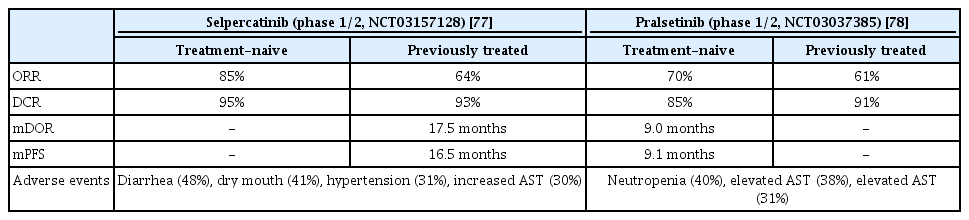
Selpercatinib is the first-developed ATP-compatible RET inhibitor. Anti-tumor activity was observed against brain metastasis in a preclinical model because it could pass through the blood-brain barrier. In a phase 1/2 study (LIBRETTO-001, ClinicalTrials.gov Identifier: NCT03157128), the selpercatinib effect was confirmed in patients who had previously received platinum-based chemotherapy and treatment-naive patients. In a previous RET-positive NSCLC patient group receiving platinum-based chemotherapy, the ORR was 64%, CR was 2%, PR was 62%, mDOR was 17.5 months, and mPFS were 16.5 months (Table 7).
The intracranial response was relatively high at 91%, CR was 27%, PR was 64% and median CNS DOR was 10.1 months. In the treatment-naive group, the ORR was 85%, and the mPFS was not measured because of a continuous response at 90% during the tracking period (median, 7.4 months). Diarrhea was the most common adverse event, followed by dry mouth, hypertension, and elevated AST levels. Grade 3 adverse events included hypertension and increased ALT level [77].
2. Pralsetinib (Gavreto)
Similar to selpercatinib, pralsetinib is an FDA-approved RET inhibitor. The efficacy was confirmed in a phase 1/2 study (ARROW, ClinicalTrials.gov Identifier: NCT03037385). The patient group that previously received platinum-based chemotherapy had an ORR of 61%, and CR and PR of 6% and 55%, respectively. The mDOR was not reached at the median follow-up from the first response. In the treatment-naive group, the ORR, CR, and PR were 70%, 11%, and 59%, respectively; mDOR was 9.0 months and the mPFS was 9.1 months in this group (Table 7). In patients with intracranial metastases, 56% had an intracranial response and three had CR. Elevated AST level was the most common adverse event, followed by elevated ALT level, construction, and neutropenia. Grade 3 or higher adverse events were neutropenia, and hypertension and anemia were also followed [78].
Conclusion
Lung cancer has a high mortality rate in Korea and worldwide [79,80]. The most common type of cancer is adenocarcinoma, which is associated with various gene mutations. New agents for adenocarcinoma have concentrated on genetic mutations previously intractable or have targeted acquired resistance brought on by earlier drug generations. Efforts to overcome drug resistance are ongoing, and more targeted agents are expected to emerge in the future.
Notes
Authors’ Contributions
Conceptualization: Lee EH, Kim EY, Chang YS, Lee SH. Writing - original draft preparation: Chung EK, Yong SH, Lee EH. Writing - review and editing: Chung EK, Yong SH, Kim EY, Chang YS, Lee SH. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a Severance Hospital Research fund for Clinical excellence (SHRC) (C-4-2022- 0268).