Transbronchial Lung Cryobiopsy for Diagnosing Interstitial Lung Disease: A Retrospective Single-Center Experience
Article information
Abstract
Background
An accurate diagnosis in patients with interstitial lung diseases (ILDs) by multidisciplinary discussion (MDD) based on histopathologic information is essential for optimal treatment. Transbronchial lung cryobiopsy (TBLC) has increasingly been used as a diagnostic alternative to surgical lung biopsy. This study aimed to evaluate the appropriate methods of TBLC in patients with ILD in Korea.
Methods
A total of 27 patients who underwent TBLC were included. TBLC procedure details and clinical MDD diagnosis using TBLC histopathologic information were retrospectively analyzed.
Results
All procedures were performed under general anesthesia with the fluoroscopic guidance in the operation room using flexible bronchoscopy and endobronchial balloon blocker. The median procedure duration was less than 30 minutes, and the median number of biopsies per participant was 2. Most of the bleeding after TBLC was not severe, and the rate of pneumothorax was 25.9%. The most common histopathologic pattern was alternative (48.2%), followed by indeterminate (33.3%) and usual interstitial pneumonia (UIP)/probable UIP (18.5%). In the MDD after TBLC, the most common diagnosis was idiopathic pulmonary fibrosis (33.3%), followed by smoking-related ILD (25.9%), nonspecific interstitial pneumonia (18.6%), unclassifiable-ILD (14.8%), and others (7.4%).
Conclusion
This first single-center experience showed that TBLC using a flexible bronchoscopy and endobronchial balloon blocker with the fluoroscopic guidance under general anesthesia may be a safe and adequate diagnostic method for ILD patients in Korea. The diagnostic yield of MDD was 85.2%. Further studies are needed to evaluate the diagnostic yield and confidence of TBLC.
Introduction
Interstitial lung diseases (ILDs) are a heterogeneous group of chronic, progressive, diffuse parenchymal lung diseases with different prognostic and therapeutic implications. An accurate diagnosis of ILD is necessary for the prediction of prognosis and optimal treatment and requires a multidisciplinary discussion (MDD) approach that includes input from clinicians, radiologists, and pathologists [1,2]. Histopathological assessment of lung tissue is critical for the diagnosis of ILD in up to 30% of patients suspected with these diseases [3,4]. Surgical lung biopsy (SLB) is the gold standard for obtaining lung tissues; however, it is invasive and associated with the risk of severe complications and mortality [5,6].
Transbronchial lung cryobiopsy (TBLC) is increasingly recognized and has been widely used as an alternative strategy for the diagnosis of ILDs [7]. Studies have shown that TBLC provides comparable diagnostic yields in the setting of MDD compared with SLB [8-11]. The important advantages of TLBC are the significantly lower rate of procedure-associated complications and mortality rate compared with SLB [12,13]. Therefore, TBLC is commonly used as a safer procedure with a comparable diagnostic yield compared with SLB [14,15]. There is no established method for performing TBLC, and it is necessary to consider the appropriate method regarding various and specific factors for each country or institution. Therefore, our aim in this study was to determine the appropriate method and safety profile of TBLC in patients with ILD. These findings may help support the use of TBLC as an alternative option to SLB in Korea.
Materials and Methods
1. Study population
This was a retrospective, descriptive, single-center study that included 27 patients with ILD who underwent TBLC at Haeundae Paik Hospital, Busan, Republic of Korea from July 2019 to December 2021. All subjects were evaluated and diagnosed by international guidelines by the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society/Latin American Thoracic Society [2,16]. Decision of performing the pathologic exam and final diagnosis was made in the MDD comprising two pulmonologists, one pathologist, two radiologists, and two rheumatologists. TBLC was performed only in patients with preference following informed consent after explanation of TBLC and SLB. The study protocol was approved by the Institutional Review Board of Haeundae Paik Hospital (approval number: 2019-08-007). The requirement for written informed consent was waived due to the retrospective nature of this study.
2. Clinical information
Clinical data for all patients were retrospectively obtained from medical records. The results of pulmonary function test and six-minute walk test (6MWT) within three months were collected. Spirometry, measurement of diffusing capacity of the lung for carbon monoxide (DLco), and plethysmography for the measurement of total lung capacity were performed according to the recommendations of the ATS/ERS; the results were expressed as percentages of normal predicted values [17-19]. The 6MWT was performed according to ATS guidelines [20]. Bronchoalveolar lavage was performed at the same time of TBLC or within three months in accordance with ATS guidelines [21]. Serum laboratory tests including autoimmune panel were collected.
3. TBLC procedure
TLBC was performed by one pulmonologist and one experienced interventional pulmonologist. All procedures were performed under general anesthesia with spontaneous ventilation through an endotracheal tube using fluoroscopy in the operation room. A flexible broncho-scope (BF-P290, 4 mm scope, Olympus, Tokyo, Japan) and a 1.7-mm or 1.9-mm cryoprobe (ERBECRYO [2], Erbe Elektromedizin GmbH, Tuebingen, Germany) were used. To locate the target lesion, the cryoprobe via bronchoscopy was advanced into a segmental bronchus and a target site 1–2 cm away from the pleura was confirmed under fluoroscopic guidance. After freezing of the cryoprobe for six seconds, the bronchoscope and cryoprobe were simultaneously pulled back, followed by immediately inflation of the endobronchial balloon for hemostasis. For the endobronchial blocker, the Fogarty catheter (4Fr, Edwards Lifesciences, Irvine, CA, USA) and the Univent tube (TCB type, Fuji Systems, Tokyo, Japan) were used. The obtained specimens were thawed in saline and pressured negatively with a syringe for one minute to prevent crush artifact and then transferred gently to formalin for fixation. The classification of bleeding severity based on the four-point scare suggested by Hetzel et al. [22] as follows: grade 0, no bleeding; grade 1, mild bleeding with self-limiting, manageable with suction alone; grade 2, moderate bleeding requiring additional intervention such as instillation of saline or transient balloon tamponade to stop bleeding; and grade 3, severe bleeding requiring additional prolonged monitoring or intensive care therapy after the procedure or if the bleeding was fatal [22]. Detection of pneumothorax was investigated using fluoroscopy during the procedure and by chest X-ray after the procedure.
4. Statistical analysis
Data are presented as frequency with percentages for categorical variables and median and interquartile range values for continuous variables. Some categorical variables (“Bleeding” and “Treatment”) include one or more values per patient, and the percentages of those variables are presented as the percentage of patients represented by each value. All statistical analyses were carried out using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
1. Baseline clinical characteristics
A total of 27 ILD patients who underwent TBLC were included in this study. The median patient age was 65.1 years (range, 63.0–71.0), and 74.1% were male. Among the patients, 74.1% were ever smokers, and the median pack-years was 40.0 (range, 26.3–48.8). Most patients (88.9%) showed a reduced DLco and more than half of the patients revealed a mild restrictive ventilatory defect. In the MDD prior to TBLC, there were no relevant underlying conditions associated with ILD, except in two patients who were diagnosed with interstitial pneumonia with autoimmune features. The clinical characteristics of the patients are summarized in Table 1.
2. TBLC details
In most patients (81.5%), the median duration of the procedure was less than 30 min, and the median number of biopsies per participant was two (Table 2). All TBLC were performed at the lower lobe (right lobe, 62.9% and left lobe, 37.1%), and the most common level of segmental bronchus was anterior basal (right) or anteromedial (left) (63.5%), followed by lateral basal bronchus (34.6%) according to the Jack-Huber nomenclature of the tracheobronchial tree [23]. The median of the largest axis diameter and smallest axis diameter of the specimens were 0.5 cm (range, 0.5–0.7 cm) and 0.3 cm (range, 0.2–0.3 cm), respectively.
Regarding complications, most bleeding after TBLC was not severe and manageable. Among three patients of severe bleeding requiring additional monitoring with use of endobronchial blockers or vasopressor, all patients were recovered without intensive care unit (ICU) admission or surgery. Seven patients (25.9%) had pneumothorax, and two patients recovered after percutaneous chest tube drain during 3 or 4 days. One patient exhibited acute exacerbation after 5 days of the procedure. There was no adverse event of pneumonia and death.
3. Histopathologic assessment and MDD
The most common pattern observed was alternative (48.2%), followed by indeterminate (33.3%) and usual interstitial pneumonia (UIP)/probable UIP (18.5%) (Table 3, Figure 1). Among the alternative patterns, the smoking-related pattern was most common (22.2%), followed by nonspecific interstitial pneumonia (NSIP) (11.1%), inhalation injury (7.4%), and others (7.4%). One patient met the histopathologic criteria of pleuroparenchymal fibroelastosis suggested by Reddy et al. [24], and one patient was diagnosed with malignancy (adenocarcinoma). In the MDD after TBLC, the most common consensus diagnoses were idiopathic pulmonary fibrosis (IPF) (33.3%) and smoking-related ILD (25.9%); four patients (14.8%) did not fulfill diagnostic criteria and were classified as unclassifiable-ILD [25,26]. Among nine patient diagnosed with IPF on final MDD, four patients were diagnosed with IPF according to the radiologic results (1 UIP and 3 probable UIP), not based on the histopathological result (4 indeterminate). Among eight patients for with non-diagnostic on clinic-radiologic evaluation (4 indeterminate for UIP, 2 probable UIP and 1 alternative pattern on high-resolution computed tomography [HRCT]), final consensus of MDD (4 smoking-related ILD, 1 inhalation injury, 1 NSIP, and 1 lung cancer) were archived on based on histopathologic results. The final diagnostic yield of MDD based on histopathologic specimens by TBLC was 85.2%, and most patients (81.5%) received anti-fibrotic agent or steroid combined with immunosuppressive agents.
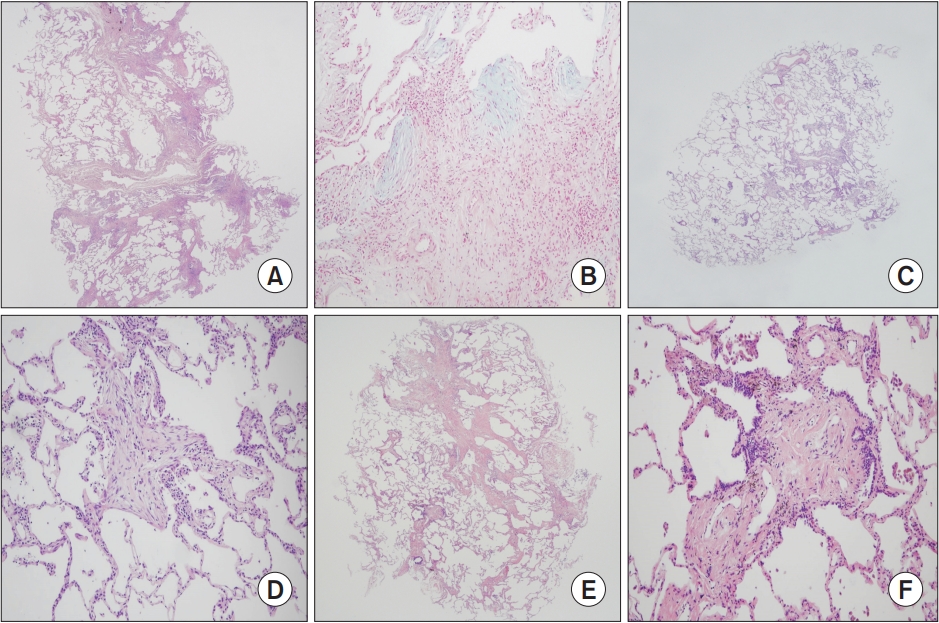
Histopathologic findings of TBLC specimens. (A) Probable UIP pattern. Patch fibrosis along the interlobular spectrum alternating normal alveoli in H&E stain and low power (×12.5). (B) Probable UIP pattern. Demonstration of the fibroblastic foci in alcian blue stain and high power (×200). (C) Cellular NSIP pattern. Well preserved alveolar architecture without paraseptal fibrosis in H&E stain and low power (×12.5). (D) Cellular NSIP pattern. Intra-alveolar plugs of fibrosis with moderate alveolar septal lymphoplasmacytic infiltrate in H&E stain and high power (×100). (E) Smoking-related pattern. Centriacinar fibrosis without involvement of the paraseptal area in H&E stain and low power (×12.5). (F) Smoking-related pattern. Temporally homogenous fibrosis and intra-alveolar pigmented macrophage accumulation in H&E stain and high power (×100). TBLC: transbronchial lung cryobiopsy; UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; H&E: hematoxylin and eosin.
Discussion
In our study, TBLC was performed under general anesthesia using flexible bronchoscopy and an endobronchial balloon blocker with the guidance of fluoroscopy. There were no severe complications requiring ICU admission or surgery. Most bleeding events were manageable with an endobronchial balloon blocker, and the incidence of pneumothorax was 25.9%. The diagnostic yield of MDD based on TBLC specimens was 85.2%.
Although TBLC was performed in a small number of patients in the current study, the complications of TBLC were mild and similar to those in previous reports [28-30]. In this study, we used the endobronchial balloon blocker in all patients, and there was no severe bleeding resulting in ICU admission or death. In a previous study addressing the influence of prophylactic bronchial blocker balloon use, the incidence of moderate to severe bleeding was significantly lower in patients who underwent TBLC with prophylactic balloon placement than those without (35.7% vs. 1.8%, p<0.001) [31]. In this study, the incidence of pneumothorax was 25.9%, and 7.4% of patients required percutaneous chest tube drain. We suggest that the guidance of fluoroscopy had an important role of reducing the incidence and severity of pneumothorax. In a study by Dhooria et al. [31] of 128 ILD patients, the incidence of pneumothorax was also significantly lower in patients who underwent the procedure with fluoroscopy compared with those without fluoroscopy (5.9% vs. 20.9%, p=0.01).
Although a standardized practice for TBLC has not yet been established, a learning curve has been suggested in previous studies. Almeida et al. [32] reported that proficiency in TBLC is achieved at approximately 70 procedures using logarithmic regression of diagnostic yield, sample length, and area for groups of consecutive patients. In a study of 512 patients with TBLC, Niwa et al. [33] reported that the incidence of pneumothorax and bleeding decreased after the 79th and 69th experiences, respectively, suggesting a learning curve of TBLC of 79 experiences. In a recent online survey on the practice of TBLC, most TBLC training (43%) was performed by self-training, followed by fellowship training and procedure course/workshops [34]. We have been preparing the setting of TBLC for one year and underwent a short-term training course at an experienced ILD center abroad twice. Therefore, we suggest that in starting TBLC, a direct training course from an experienced center or systematic training course considering specific environmental factors might be helpful to reduce the learning curve and improve patient safety.
The diagnostic yield of TBLC and classification rate of unclassifiable-ILD in this study were similar to those in previous studies [26,30,35]. Histopathologic classification of probable UIP was diagnosed in 18.5% of patients, and there was no UIP pattern. And four patients were diagnosed with IPF according to the radiologic pattern, not based on the histological result. We assumed that the absence of UIP in histopathologic analysis might be explained because patients with relatively early stage and who do not have UIP pattern on HRCT were included in this study. Also, it might be the result reflecting the limitations of TBLC in this study, which were related to the small number and size of histopathologic samples, and lack of experiences in histopathological analysis. Among nine patients finally diagnosed with IPF, four patients (44.4%) were diagnosed with four histologic classifications of indeterminate including three probable UIP and one indeterminate on radiologic diagnosis using HRCT. In line with previous studies addressing the important role of MDD, MDD was necessary to in- crease the diagnostic confidence and yield of TBLC in patients with ILD [36,37].
This study has some limitations. First, this was a retrospective study conducted in a single center with a small number of patients. However, the safety profiles of TBLC and diagnostic yield of MDD in this study were similar to those in previous reports. Second, the diagnostic yield using TBLC histopathologic diagnosis in this study was not sufficiently powered because there was no control group of SLB and the MDD did not have sufficient expertise with TBLC. Further study is warranted to prove the diagnostic yield of TBLC by interdisciplinary diagnosis with a more experienced MDD group, considering the ethical concerns of performing SLB and TBLC simultaneously. Third, all procedures were performed at the lower lobe and same lobe with a relatively small number of specimens. However, the recommended minimum number of biopsy is two, and all biopsies were conducted at a different site even in the same lobe. If the safety of the procedure is secured, more numbers of biopsies might be useful to improve the diagnostic yield. Fourth, all the procedures were performed with relatively smaller size of cryoprobe. However, the diameter of TBLC specimens in this study was not small, and in previous study to compare the diagnostic yield according to cryoprobe size between 1.9 mm and 2.4 mm, no significant differences were observed with respect to the diagnostic yield and safety profile [38].
In conclusion, our study indicates that the TBLC method under general anesthesia with flexible bronchoscopy and endobronchial balloon blocker with the guidance of fluoroscopy might be safe and adequate for patients with ILDs in Korea. The diagnostic yield of MDD based on histopathologic diagnosis by TBLC was 85.2%.
Notes
Authors’ Contributions
Conceptualization: Park JH, Jang HJ, Lee JH. Methodology: Park JH, Kim HK, Jang HJ. Formal analysis: Park JH, Choi HE, Kim IH. Data curation: Jang JH, Kim JY, Han JY, Kim DS, Kang E. Validation: Kim HK, Choi HE, Han JY, Kang MK. Investigation: Park JH, Kang MK, Han JY, Kim DS, Lee JH. Writing - original draft preparation: Park JH, Lee JH. Writing - review and editing: Park JH, Jang JH, Kim HK, Jang HJ, Lee S, Kim SH, Kim JY, Choi HE, Han JY, Kim DS, Kang MK, Kang E, Kim IH, Lee JH. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Acknowledgements
The authors would like to thank Da Som Lee and Eun Gyeong Tak (clinical nurse specialists in the Department of Critical Care and Pulmonology in Haeundae Paik Hospital) and Young Ju Heo (Coordinator of the Haeundae Paik Hospital Interstitial Lung Disease Center) for their contributions and efforts.



