The Long-term Efficacy of Domiciliary Noninvasive Positive-Pressure Ventilation in Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
Background
We evaluated the long-term effects of domiciliary noninvasive positive-pressure ventilation (NIPPV) used to treat patients with chronic obstructive pulmonary disease (COPD).
Methods
Databases were searched to identify randomized controlled trials of COPD with NIPPV for longer than 1 year. Mortality rates were the primary outcome in this meta-analysis. The eight trials included in this study comprised data from 913 patients.
Results
The mortality rates for the NIPPV and control groups were 29% (118/414) and 36% (151/419), suggesting a statistically significant difference (risk ratio [RR], 0.79; 95% confidence interval [CI], 0.65–0.95). Mortality rates were reduced with NIPPV in four trials that included stable COPD patients. There was no difference in admission, acute exacerbation and quality of life between the NIPPV and control groups. There was no significant difference in withdrawal rates between the two groups (RR, 0.99; 95% CI, 0.72–1.36; p=0.94).
Conclusion
Maintaining long-term nocturnal NIPPV for more than 1 year, especially in patients with stable COPD, decreased the mortality rate, without increasing the withdrawal rate compared with long-term oxygen treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible limitation of air inflow induced by damage to the airway and lung parenchyma due to chronic inflammation [1,2]. Chronic hypoxia induced by long-term airflow limitation induces pulmonary vasoconstriction, polycythemia, right heart failure, and multi-organ dysfunction [1,2]. In particular, the muscle tone and respiratory muscle movements are reduced during nighttime rapid eye movement (REM) sleep [3]. Thus, ventilatory dysfunction is further exacerbated in patients with COPD in sleep state. In patients with COPD, hypoxic and hypercapnic states due to ventilatory disturbance at night are not fully resolved even during the daytime, resulting in poor prognosis [3].
Non-pharmacologic treatments for COPD include smoking cessation, oxygen therapy, rehabilitation, pneumococcal vaccination, and noninvasive positive-pressure ventilation (NIPPV) [1,2]. The use of NIPPV in patients with COPD prevents deterioration of patient’s condition by assisting lung ventilation [4]. Although NIPPV treatment for acute respiratory failure resulting from COPD exacerbation improves mortality outcomes [5,6], the studies report a wide range of mortality in patients treated with domiciliary NIPPV [7-14]. A study using nasal NIPPV to treat patients with COPD found improvement in the ventilation index such as increased partial pressure of oxygen (PaO2) and decreased partial pressure of carbon dioxide in the arterial blood (PaCO2) [4]. In randomized trials of patients with COPD treated with NIPPV, the sleep quality or quality of life (QOL) improved, but not the survival rate or pulmonary function [12,14]. Other studies of patients with COPD treated with NIPPV showed improved survival outcomes [11,13,15]. A meta-analysis [16] of patients with COPD treated with NIPPV suggested that high baseline PaCO2 levels are associated with good outcomes in terms of survival and hospital readmission.
Although meta-analyses of the effect of NIPPV on COPD associated with chronic respiratory failure have been conducted [16,17], few studies have analyzed the long-term efficacy of NIPPV in such patients. In the present study, we evaluated the effects of domiciliary NIPPV lasting more than a year on mortality rate, differences in QOL, admission rates, and treatment withdrawal of patients with COPD.
Materials and Methods
Databases were searched to identify randomized controlled trials (RCTs) involving long-term (more than 1 year) NIPPV treatment of COPD. The following search terms including medical keywords and headings were employed: “pulmonary disease, chronic obstructive”, or “chronic obstructive lung disease”, or “emphysema”, and “respiratory therapy”, or “noninvasive”, or “bi-level”. The detailed search strategy for retrieval method is included as a supplementary file (see Supplementary Table S1). Two researchers judged a number of articles on the feasibility of this study, and in case of conflict, the two discussed and agreed. We included RCTs comparing domiciliary NIPPV with usual therapy, including long-term oxygen therapy, for the management of adult patients (18 years of age or above) with COPD.
We requested raw data for all included studies to analyze the mortality and outcomes of patient subgroups. Two authors replied, and one sent the materials in response to our request.
Two researchers independently analyzed data from the studies, including study population, year of publication, study design, NIPPV details (including duration of the study, enrollment criteria, baseline forced expiratory volume in 1 second [FEV1], and PaCO2 level), mean inspiratory positive airway pressure (IPAP) level, actual duration of NIPPV, treatment of the control group, and clinical outcomes (including mortality, QOL, admission rate, and withdrawal rate). Disagreements regarding the interpretation of data were resolved by consensus between the two investigators. The primary outcome was the all-cause mortality rate in patients with COPD. Patients were subdivided into two groups based on status at time of enrollment: (1) stable and (2) admitted to a hospital at the time of enrollment and subsequently discharged.
The methodological quality and risk of bias were assessed using a modified version of the Cochrane risk-of-bias instrument. If the two researchers disagreed about the quality and risk of bias of enrolled studies, the differences were resolved through discussion and an agreement was reached. Because NIPPV equipment was always visible to patients and medical staff, the study cannot be completely blinded to study participants. Therefore, all included studies had a high degree of performance bias. However, all studies were judged to be at low risk of bias in that the outcome measurements were not influenced by a lack of blinding of the study personnel.
We studied outcome data at the trial level and performed statistical calculations using Review Manager software (RevMan version 5.3, Nordic Cochrane Centre, Cochrane Collaboration, 2011). Continuous outcomes were reported as mean differences (a measure of absolute change), and binary outcomes were reported as risk ratios (RRs) [18]. All statistical results were two-sided. We considered p<0.05 to be statistically significant for all analyses with 95% confidential intervals (CIs) [18]. We reported the summary results for all individual trials. Further, we evaluated the between-study heterogeneity of each outcome using the I2 statistic. We regarded statistical heterogeneity as low if I2=25%–49%, moderate if I2=50%–74%, and high if I2 ≥75% [18].
Results
We identified 18,408 citations based on a search of electronic databases. Thirteen citations were retrieved for more detailed evaluation, and eight of those studies met the criteria for inclusion in our review (Figure 1). Two researchers reached a perfectly consistent decision through discussion with each other regarding inclusion of all studies. The eight trials [7-14] (Table 1) included in this study comprised data from 913 patients (median, 113 patients per trial; range, 47–201 patients). The follow-up periods in the included studies were 12–24 months.
Although there was no mortality outcome in the study of Duiverman et al. [10], we included it in our meta-analysis because it included withdrawal rates and QOL parameters.
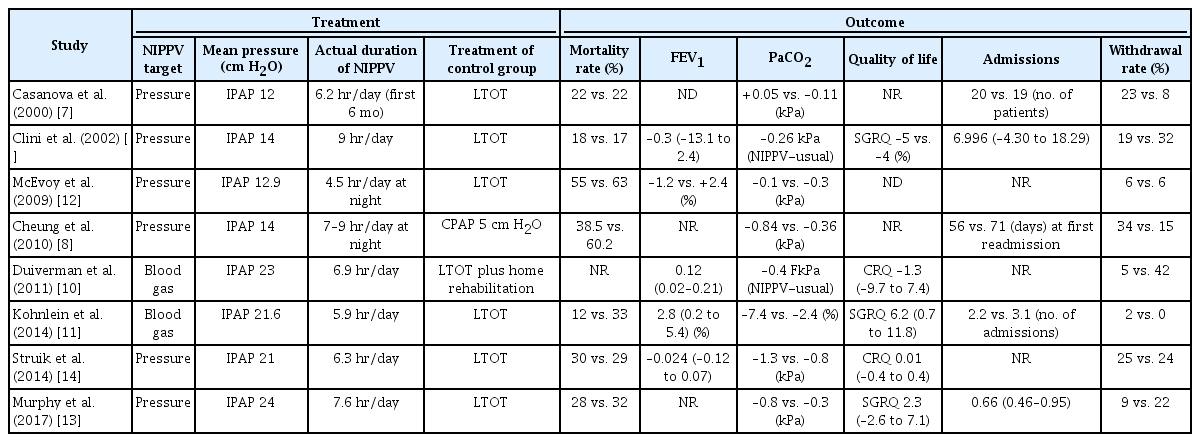
The baseline characteristics of the included studies are presented in Table 1. Five studies involved stable COPD patients, and three included COPD patients who were discharged after being hospitalized. Most of the patients were over 60 years of age and had severe airflow obstruction with a mean predicted FEV1 <50%. The treatments, outcomes, and withdrawal rates documented in the studies are presented in Table 2. Data pertaining to mortality, FEV1, QOL, admission, and withdrawal were pooled. The NIPPV target was pressure in six studies and blood gas in two studies. Four studies after 2011 set IPAP as high as 20 or higher. The actual application time of NIPPV in each study ranged from 4.5 hours to 9 hours. FEV1 compared the two groups as a ratio or percent difference, and the degree of PaCO2 decrease was compared in kPa or the difference between the two groups was described at the end of the study. The methodological quality of included trials is presented in Supplementary Figure S1.
Figure 2 presents mortality rates for the included studies [7-9,11-14], with the exception of Duiverman et al. [10]. The mortality rates for the NIPPV and control groups were 29% (118/414) and 36% (151/419). This difference was statistically significant (RR, 0.79; 95% CI, 0.65–0.95; p=0.01). There was statistical heterogeneity among the trials that provided mortality data (p=0.13, I2=40%). The RRs for mortality in the individual RCTs are presented in Figure 2.

Forest plot describing the effect of noninvasive positive-pressure ventilation (NIPPV) on all-cause mortality, and the mortality rate according to the status of patients with chronic obstructive pulmonary disease (COPD) [7-9,11-14]. The vertical line depicts the equivalence in mortality rates between the two groups (NIPPV vs. control), and horizontal lines correspond to the 95% confidence intervals (CIs). The size of each square represents the proportion of information provided by each study.
The results of subgroup analyses are summarized in Figure 2. The mortality rates in the four trials that included patients with stable COPD [7,9,11,12] were reduced after exposure to NIPPV (RR, 0.71; 95% CI, 0.56–0.91; p=0.006). All-cause mortality rates in the three trials involving COPD patient post-hospital did not differ between the NIPPV and control groups (RR, 0.90; 95% CI, 0.67–1.21; p=0.48).
The results of admission and acute exacerbation in studies [7,8,14] are summarized in Figure 3. There was no difference in admission exacerbation (RR, 0.99; 95% CI, 0.78–1.26; p=0.94) and acute exacerbation (RR, 0.83; 95% CI, 0.59–1.15; p=0.26) between the NIPPV and control groups (Figure 3).

Forest plot depicting the effect of noninvasive positive-pressure ventilation (NIPPV) on admission (A) and acute exacerbation (B) [7,8,14]. The vertical line depicts the equivalence in mortality rates between the two groups (NIPPV vs. control), and the horizontal lines correspond to the 95% confidence intervals (CIs). The size of each square represents the proportion of information provided by each study.
QOL was analyzed in studies [10,14] that reported Chronic Respiratory Questionnaire (CRQ) scores. There was no difference in QOL between the NIPPV and control groups (standardized mean difference, –0.037; 95% CI, –0.34 to 0.29; p=0.85) (Figure 4).
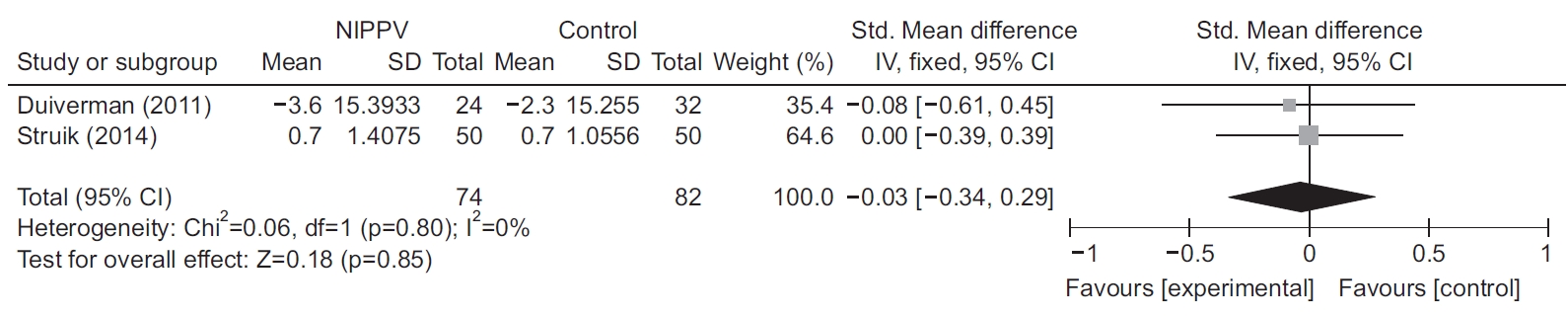
Forest plot depicting the effect of noninvasive positive-pressure ventilation (NIPPV) on the Chronic Respiratory Questionnaire [10,14]. The vertical line depicts the equivalence in mortality rates between the two groups (NIPPV vs. control), and the horizontal lines correspond to the 95% confidence intervals (CIs). The size of each square represents the proportion of information provided by each study. SD: standard deviation.
All included RCTs reported data concerning withdrawal or dropout in the NIPPV and control groups (Table 2, Figure 5). There was no significant difference in the dropout rate between the two groups (RR, 0.99; 95% CI, 0.72–1.36; p=0.94).
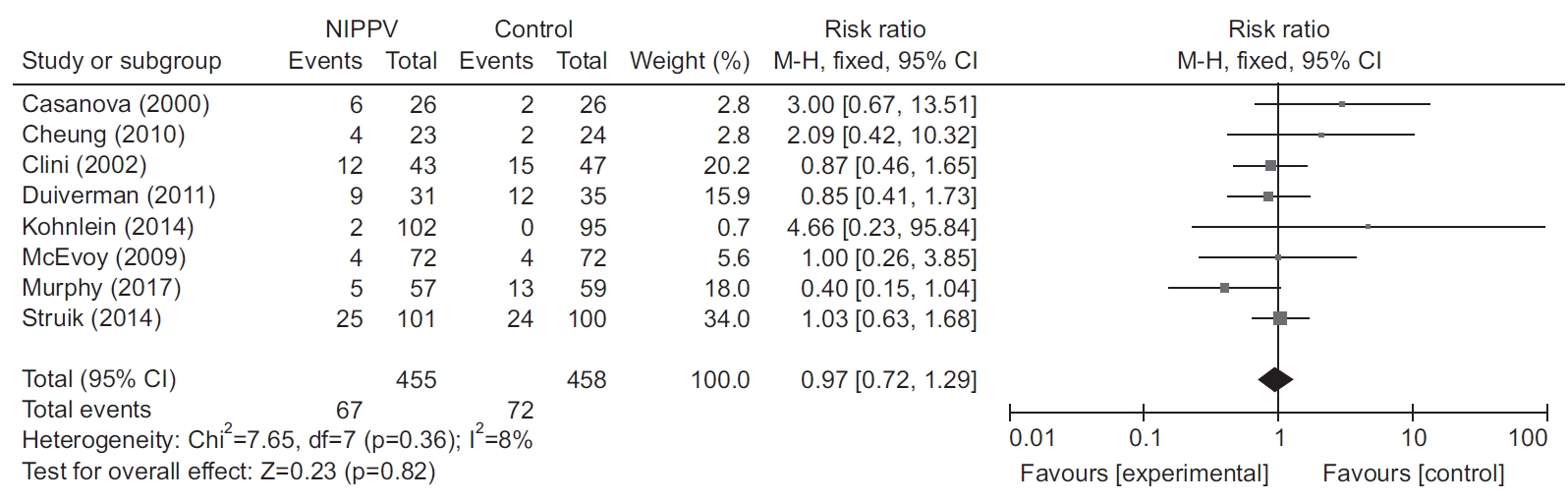
Forest plot depicting the effect of noninvasive positive-pressure ventilation (NIPPV) on withdrawal rates according to mean level of inspiratory positive airway pressure (IPAP) (≥20 cm H2O) [7-14]. The vertical line depicts the equivalence in mortality rates between the two groups (NIPPV vs. control), and the horizontal lines correspond to the 95% confidence intervals (CIs). The size of each square represents the proportion of information provided by each study.
Discussion
The main finding of our meta-analysis was that the mortality of patients with COPD was reduced after treatment with domiciliary NIPPV for more than a year. Furthermore, the rate of withdrawal was not high in the NIPPV group, indicating that patients adapted relatively well to NIPPV.
The hypoxic and hypercapnic conditions of COPD with chronic ventilatory dysfunction lead to pulmonary vasoconstriction, polycythemia, and multiple organ dysfunction [1,2]. Therefore, the long-term use or effects of home oxygen improves survival of COPD patients19. However, oxygen therapy alone failed to facilitate the ventilation of patients with COPD, and occasionally, the administration of oxygen aggravated the ventilation and perfusion ratio (VQ) mismatch and increased carbon dioxide levels in the patients [19]. Because NIPPV improves ventilation in patients, it improves both hypoxic and hypercapnic status and prevents VQ mismatch in the lungs of patients treated with oxygen alone. Especially, the REM sleep state induces muscle paralysis and reduces the respiratory muscle tone in healthy people [3]. Patients with COPD exhibit a serious progression of this ventilatory dysfunction [3]. The exacerbation of hypoxia and hypercapnia during nighttime is not fully restored during daytime [3]. The deterioration in progress is accelerated in patients with COPD [3]. The improved ventilation in patients with COPD following long-term use of NIPPV may increase survival and QOL and reduce comorbidities or acute exacerbation. A small-population study reported that domiciliary NIPPV improved the ventilatory index, quality of sleep, and QOL in patients with COPD [4]. However, several randomized studies have reported conflicting results, particularly in terms of the mortality rate, ventilatory index, and QOL score [7-14]. Recently, Murphy et al. [13] suggested that home NIPPV prolonged the time to readmission or death within a 12-month period among patients with persistent hypercapnia following acute exacerbation of COPD.
A systematic review by Dretzke et al. [16] included both RCTs and retrospective studies of domiciliary NIPPV in COPD, regardless of NIPPV duration. The review suggested that domiciliary NIPPV in COPD did not reduce mortality but did reduce mortality in stable COPD. Our analysis of all included studies involving patients with stable or post-hospital COPD showed decreasing mortality rates in patients receiving NIPPV longer than 12 months. Contrary to the results of Dretzke et al. [16], the improved survival rate of patients in the NIVtreated group in our study can be attributed to the omission of observation studies and inclusion of the latest study showing good survival rates. The subgroup analysis of COPD status showed that the mortality rates were not decreased among post-hospital COPD patients using NIPPV based on the analysis of a small number of studies. A recent study [13] included only COPD patients with persistent hypercapnia in post-hospital status, with improved mortality following home NIPPV. In the previous studies of home NIPPV involving patients of posthospital status, the efficacy of NIPPV was not obvious because subjects with chronic ventilation dysfunction were combined with those manifesting good lung function after acute exacerbation. Although patients in the post-hospital group showed elevated PaCO2 due to temporary exacerbation, the effect of NIPPV ventilation is not clear because the baseline PaCO2 in stable post-hospital patients after recovery may not be high. In the other meta-analysis [16], the group with high PaCO2 in the post-hospital group showed improved survival rate. The Kohnlein’s study [13] reported a positive effect on mortality when patients with a high baseline PaCO2 were enrolled in the posthospital group.
In the four studies investigating high IPAP after 2011, the reduction of PaCO2 was greater in the NIPPV group, and the previous study showed mixed results of PaCO2 suggesting that high IPAP can lead to effective ventilation and reduction of PaCO2.
Analyses of additional outcomes associated with the long-term use of NIPPV, including QOL, lung function, and acute exacerbation, were difficult because of variation in the tools used for evaluation in the studies. Improvements in lung function in the included studies were assessed using the absolute FEV1 value, the predicted value [12], or the mean difference [10,14]. It was also very difficult to assess the readmission rate or acute exacerbation rate in COPD patients. Various studies presented readmission data according to the number of readmissions per patient [7], the total number of readmissions during the study period [11], the time to initial readmission [8], and the mean difference [13]. Further studies are required to analyze the efficacy of long-term NIPPV in COPD based on standard assessment tools for acute exacerbation, readmission, lung function, and QOL. Our study showed that QOL, as assessed by the CRQ, did not differ between the NIPPV and control groups. Additional studies investigating QOL improvement in COPD patients treated with NIPPV are needed as the benefits of intervention vary depending on the equipment used. The cost of NIPPV also needs further analysis.
A recent meta-analysis reviewed the effect of long-term NIPPV on stable hypercapnic COPD [20]; however, it included only stable patients with COPD manifesting hypercapnia, and lasted more than 3 months [20]. NIPPV may be useful in patients with persistent hypercapnia after acute exacerbation [13]. A meta-analysis of studies reporting survival in COPD following exposure to NIPPV longer than one year has yet to be reported.
NIPPV appears to be more difficult to adjust to than oxygen therapy for patients [21]. However, we did not find a difference in withdrawal rates between the NIPPV and control groups after more than a year, suggesting that patients with COPD adapted well to NIPPV over a long time, especially after adjustment.
The present analysis has several limitations. First, the included trials were somewhat diverse, given the differences in inclusion criteria, COPD severity, NIPPV duration, ventilation strategies, and associated treatments. We requested raw data for the included studies to analyze subgroups of patients and assess the settings employed by each study. Unfortunately, our request received either no response or a refusal. Second, it is likely that we did not include all of the relevant evidence because we limited our analysis to articles written in English. Third, the small number of available trials may have led to an underestimation of the heterogeneity and reduced the precision of our pooled-effect estimates.
Our systematic review demonstrated that maintaining longterm nocturnal NIPPV for more than 1 year in COPD patients, especially stable COPD, decreased the mortality rate, without increasing the withdrawal rate compared with long-term oxygen treatment. Further research in the form of large RCTs of post-hospital COPD patients with persistent hypercapnia is warranted.
Notes
Authors’ Contributions
Conceptualization: Sim YS. Methodology: Park SY, Park J. Formal analysis: Park DA. Software: Sim YS. Validation: Yoo KH, Park YB, Rhee CK. Investigation: Park HY. Writing - original draft preparation: Park SY, Sim YS. Writing - review and editing: Hwang YI. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Medical subject headings and keywords in databases.
Risk of bias using a modified version of the Cochrane risk-of-bias instrument.