Retrospective Analysis of Long-Term Survival in Very Elderly (Age ≥80) Critically Ill Patients of a Medical Intensive Care Unit at a Tertiary Care Hospital in Korea
Article information
Abstract
Background
The purpose of this study was to evaluate the long-term survival rates of very elderly (age ≥80) critically ill patients admitted to a medical intensive care unit (MICU) at a regional tertiary-care hospital in Korea.
Methods
We retrospectively analyzed data from patients who survived after discharged from the MICU of our hospital. Survival rates at 90 days, 1 year, 2 years, and 3 years were assessed between patients age ≥80 and those age <80. Survival status was evaluated using the National Health Insurance Service data.
Results
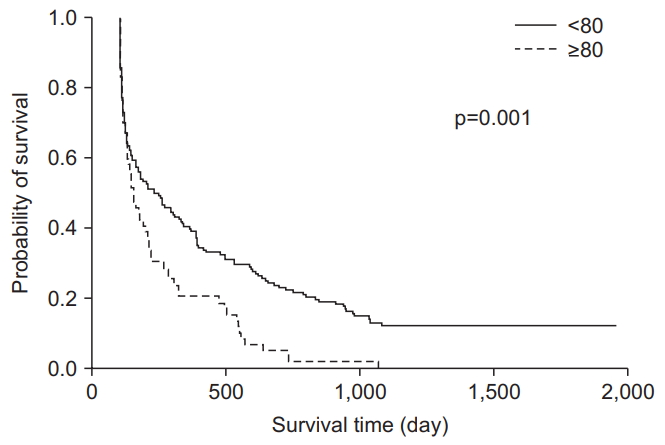
A total of 468 patients were admitted, 286 (179 males, 97 females; mean age, 70.18±13.2) of whom survived and were discharged soon after their treatment. Among these patients, 69 (24.1%) were age ≥80 and 217 (75.9%) were age <80. The 90-day, 1-year, 2-year, and 3-year survival rates of patients age ≥80 were significantly lower than those in patients age <80 (50.7%, 31.9%, 15.9% and 14.5% vs. 68.3%, 54.4%, 45.6%, and 40.1%, respectively) (p<0.01). The Kaplan-Meier survival curves showed significantly lower survival rates in patients age ≥80 than in those age <80 (p=0.001).
Conclusion
The poor rates of long-term survival in very elderly (age ≥80) and critically ill patients admitted to an ICU should be considered while managing and treating them.
Introduction
The worldwide population is rapidly aging. Consequently, the number of elderly patients admitted to intensive care units (ICUs) is increasing as is the number of elderly patients age >80 years [1,2]. According to data reported by Lim et al. [3], the number of ICU patients age ≥80 years increased from 12.8% in 2005 to 20.7% in 2014 in Korea. A recent multicenter study in Korea has also reported that 22.1% of patients admitted to ICUs are elderly patients age over 80 years [4].
In a recent study of elderly patients admitted to ICUs, the authors reported that ICU and in-hospital mortality rates do not differ significantly between patients aged more than 80 years and those younger than 80 years. The mortality rates of the ICU patients were 34.9% for patients age >80 years vs. 39.5% for those age ≤80 years [5]. However, there have been few studies conducted on the long-term survival of patients admitted to ICUs in Korea. Recent study by Heo et al. [6] reported long-term mortality according to age in patients admitted ICU using National Health Insurance data. In contrast, there is a relatively large amount of research data available outside of Korea. In one study in France, 2-year and 3-year survival rates of patients admitted to medical ICU for patients age >80 were 33% and 29%, respectively [7].
Because few studies have been conducted on the long-term survival of critically ill patients age >80 admitted to ICUs in Korea, the purpose of this study was to investigate the long-term survival and factors associated with mortality of such patients in the ICU of a single regional tertiary hospital in Korea.
Materials and Methods
All critically ill patients admitted to the medical ICU (MICU) at a single regional tertiary hospital (total hospital beds, 890; MICU beds, 13–15) between December 2011 and May 2014 were retrospectively studied. Admissions were determined by attending physicians according to general ICU admission criteria. Patient demographic and clinical characteristic data were prospectively collected and analyzed retrospectively. The following data were analyzed: sociodemographic data, including age, sex, body mass index (BMI), and chronic underlying disease; the reasons for admission; severity of illnesses, as measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) [8] and Sequential Organ Failure Assessment (SOFA) [9] systems at admission; duration of mechanical ventilation (MV); length of ICU and in-hospital stays, and outcome data, including survival status in ICU and at in-hospital. Patients were excluded if they were admitted due to acute coronary syndrome, drug overdoses, or acute cerebrovascular disease and age less than 18 years.
Weaning failure is defined as the failure to pass a spontaneous breathing trial or the need for reintubation within 48 hours following extubation and when blood transfusion is restricted to packed red cell transfusion.
Survival at 90th day, 1 year, 2 years, and 3 years were evaluated in patients who were admitted to the ICU and discharged from the hospital. The survival status of a patient was assessed based on National Health Insurance Corporation’s medical insurance standards. A patient’s death was evaluated by the cessation of medical insurance, and date of death was determined as the date of cessation. This study was approved by the institutional review board of the hospital (GNUCH-2018-10-011). The patients’ informed consent for the study was waived due to the retrospective nature of the study.
The data are presented as mean±standard deviation and median with an interquartile range. Categorical data are presented as frequencies and percentages. Independent t test (continuous variables) and chi-square test (categorical variables) results were compared between patients age ≥80 patients and those age <80. Survival curves were estimated by the Kaplan-Meier method, and with a log-rank test. Multivariate Cox proportional hazard regression models were used to evaluate factors associated with mortality in patients at 1 year after discharge. We choose the factor when univariate analysis showed p-value less than 0.05 or factor assumed with cause of death. A p-value of <0.05 was considered to be statistically significant for all tests. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) for Windows (Microsoft Corporation, Redmond, WA, USA).
Results
1. Baseline clinical characteristics of the patients who survived
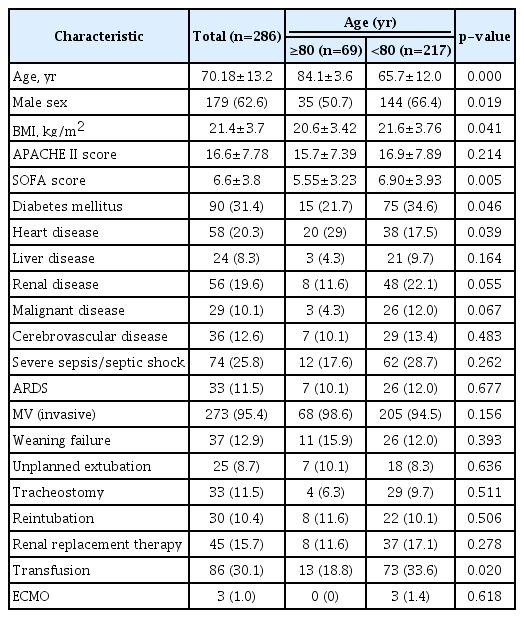
A total of 468 patients (306 male, 162 female; mean age, 68.6±13.9 years) were enrolled. Among these, 286 patients (179 male, 97 female; mean age, 70.18±13.2 years) survived and were discharged. The baseline characteristics were listed in Table 1.
2. Comparison of clinical characteristics between patients age ≥80 and those age <80
Among all patients survived, 69 (24.1%) were age ≥80 and 217 (75.9%) were age <80. There were more males among patients age <80 than among those age ≥80 (66.4% vs. 50.7%, p=0.019). BMI and SOFA scores at admission were higher in patients age <80 than in those age ≥80 (BMI: 21.6±3.76 vs. 20.6±3.4, p=0.041; SOFA score: 6.9±3.93 vs. 5.55±3.23, p=0.005). Among underlying diseases, diabetes was more common in patients age <80 than in those age ≥80 (34.6% vs. 21.7%, p=0.046). Moreover, heart disease was less common in patients age <80 than in those age ≥80 (17.5% vs. 29%, p=0.039). Transfusion requirements were more common in patients age <80 than in those age ≥80 (41.4% vs. 27.4%, p=0.02).
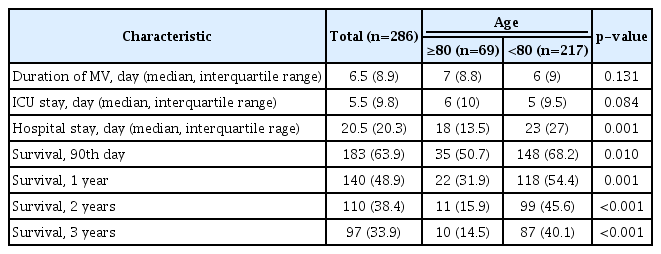
3. Comparison of the clinical outcomes and the differences in survival rates between patients age ≥80 and those age <80
Regarding clinical outcomes, the durations of hospital stay were longer for patients age <80 than for those age ≥80 (27.95±39.63 days vs. 18.17±15.45 days, p=0.001). The 90-day, 1-year, 2-year, and 3-year survival rates of patients age ≥80 were 50.7%, 31.9%, 15.9%, and 14.5%, respectively, and the corresponding survival rates in patients age <80 were 68.3%, 54.4%, 45.6%, and 40.1%, respectively (p<0.05) (Table 2). The Kaplan-Meier curves and log-rank tests for 1-year survival of patients age ≥80 showed significantly lower survival rates than those age <80 (p=0.001) (Figure 1).
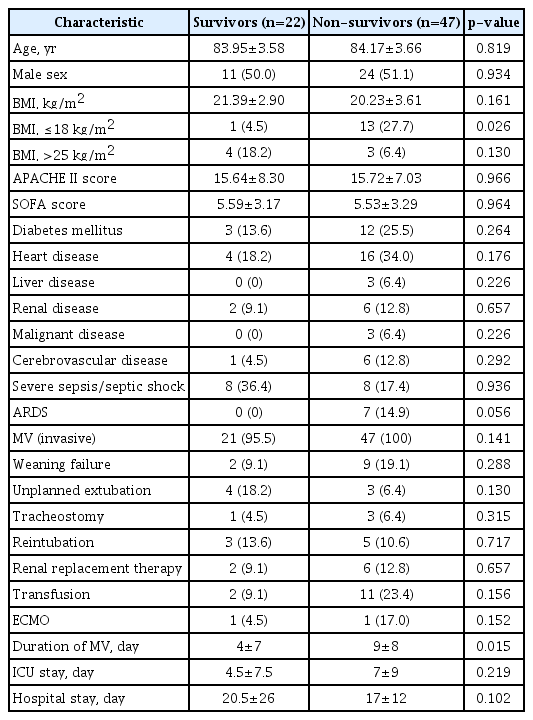
4. Comparison of clinical characteristics between survivors and non-survivors 1 year after discharge among the 69 patients age ≥80
Characteristics of survivors and non-survivors 1 year after discharge among the 69 patients age ≥80 are summarized in Table 3. The proportions of males and BMI scores were not significantly different between survivors and non-survivors. However, the proportion of BMIs ≤18 was significantly different between survivors and non-survivors (4.5% vs. 27.7%, p=0.026). No difference was found between APACHE II and SOFA scores at admission between survivors and non-survivors. Regarding underlying disease and the cause of admission, the proportion of patients presenting with severe sepsis and septic shock was not significantly different between survivors and non-survivors. Although ICU and hospital stays were not significantly different between survivors and non-survivors, the durations of MV were significantly longer in non-survivors than in survivors (5.67±4.53 vs. 9.66±8.52, p=0.015). The MV weaning failure and unplanned extubation were not significantly different between survivors and non-survivors. Requirements for renal replacement therapy, extracorporeal membrane oxygenation, and transfusions were not significantly different between survivors and non-survivors.
5. Factors associated with mortality in patients 1 year after discharge
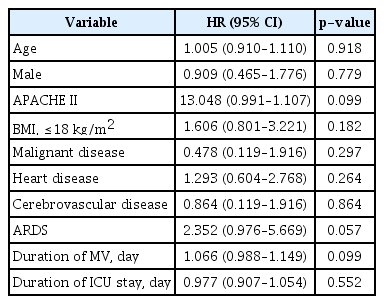
The sociodemographic data, including age, sex, BMI, underlying disease, severity of illnesses and durations of MV, and length of ICU stay were not associated with a risk of mortality at 1 year after discharge among 69 patients age ≥80, according to the multivariate Cox regression analysis of hazard ratios (Table 4).
Discussion
This study evaluated long-term survival in critically ill patients admitted to the ICU and showed poorer long-term survival in patients age ≥80 than in those age <80. Despite the increasing number of very elderly patients admitted to ICUs [3-5], there have been few reports regarding long-term survival of the very elderly and critically ill patients in Korea. A recent nationwide cohort study reported that, during the study period from 2003 to 2010, the proportion of patients age ≥70 had increased by an average of 3.6 years per study year, with 90-day, 1-year, and 3-year mortality rates in patients age ≥80 patients being 36.1%, 50.7%, and 65.9%, respectively [6]. In contrast to the lack of published data in Korea, there have been multiple studies investigating the long-term outcomes in very elderly and critically ill patients admitted to ICUs in other countries. Roch et al. [10] have investigated the 2-year mortality of ICU patients age >80 in France. Their results showed that, of 133 patients over 80 years old, 1-year and 2-year mortality rates after admission were 72% and 78%, respectively. One study conducted in Norway reported a mortality rate of approximately 60% for patients age >80 in a mixed ICU [11]. Overall, 40%–60% mortality rates have been reported in previous studies [12-18], similar to results of the present study.
Factors associated with long-term mortality in the very elderly and critically ill patients have been studied. Roch et al. [10] have studied factors associated with mortality at 2 years after hospital discharge from medical ICUs in patients age >80. They reported that more severe underlying diseases as classified by McCabe score [19] and higher results of the simplified Acute Physiologic Score II representing more severe critical illnesses were independently associated with the 2-year mortality rate after hospital discharge. Similarly, Torres et al. have reported that comorbid illness scored with the Charlson Comorbidity Index [20] was the strongest predictor of long-term survival in critically ill older patients. In a recent Korean study assessing long-term outcomes in ICU patients, higher comorbidity scores and male sex were associated with long-term higher mortality rates in these patients [6]. In other studies evaluating the factors associated with long-term mortality in elderly critically ill patients, Lown et al. [21] have reported that, in addition to age, the need for MV and the presence of acute renal failure are associated with mortality within 12 months after discharge in patients age >80. Regarding causes of admission, respiratory failure, and head injuries have been reported as predictors of 1-year mortality in a mixed ICU population of patients age 80 [11]. Other studies have demonstrated that low hemoglobin and high creatinine concentrations are independent risk factors for long-term mortality [12,13]. Some studies have reported that the duration of ICU stay may be associated with long-term mortality. Laupland et al. [22] have reported that staying in the surgical and cardiovascular ICUs for more than 14-day results in high mortality rates than shorter ICU stays. Longer ICU stay may present poor performance status at the time of discharge. Thus, it might be associated with poor long-term outcomes of critically ill patients.
Quality of life is a very important aspect in surviving patients after ICU discharge. We tend to expect a poor quality of life for critically ill patients, especially for the very elderly population. One study assessing 278 patients age ≥80 who were admitted to ICUs showed that quality of life evaluated using the EuroQol-5 Dimension (EQ-5D) questionnaire revealed a significant decrease with age, including increasing problems with mobility, self-care, and activities of daily living [17]. In addition, Pavoni et al. [14] have demonstrated that medical and orthopedic patients have poorer quality of life than surgical patients.
This study has some limitations. First, it was conducted using a single hospital’s data and limited to medical ICU patients. Second, we were unable to determine the quality of life or assistance given in nursing care facilities after discharge. Third, whether or not patients had died or survived was assessed only based on medical insurance data. Therefore, the exact time of death might have been somewhat biased in this study. Fourth, as described above, functional status at the time of admission rather than severity of illness is more predictable and an important factor to evaluate long-term outcomes. However, we assessed only the underlying disease rather than the general function status.
Despite these limitations, our study is important in that it assesses the long-term survival in very elderly and critically ill patients admitted to the ICU. This information is especially important in light of the growing elderly population in Korea. However, a future nationwide study is necessary to determine factors associated with long-term outcomes and quality of life.
Notes
Authors’ Contributions
Conceptualization: Kim HC. Methodology: Lee SH, Kim HC. Formal analysis: Kim HC. Data curation: Lee SH, Kim HC. Software: Lee SH, Kim HC. Validation: Lee SH, Kim HC. Investigation: Lee SH, Kim JY, Kim TH, Ju SM, Yoo JW, Lee SJ, Cho YJ, Jeong YY, Lee JD, Kim HC. Writing - original draft preparation: Lee SH. Writing - review and editing: Kim HC. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.