2. Kim IS, Jee SH, Ohrr H, Yi SW. Effects of smoking on the mortality of lung cancer in Korean men. Yonsei Med J 2001;42:155-160. PMID:
11371101.


3. The Forth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. 2009. cited 2011 Oct 23. Seoul: Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention; Available from:
http://knhanes.cdc.go.kr.
4. Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000;2:19-37. PMID:
11072438.



5. Zbikowski SM, Swan GE, McClure JB. Cigarette smoking and nicotine dependence. Med Clin North Am 2004;88:1453-1465. xPMID:
15464107.


6. Tonstad S. Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs 2006;21:433-436. PMID:
17293731.


7. Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-1568. PMID:
16908788.


8. Tonstad S. Varenicline for smoking cessation. Expert Rev Neurother 2007;7:121-127. PMID:
17286546.


9. Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB Jr, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 2007;29:1027-1039. PMID:
17692719.


10. Kang YS, Choi SY, Ryu E. The effectiveness of a stress coping program based on mindfulness meditation on the stress, anxiety, and depression experienced by nursing students in Korea. Nurse Educ Today 2009;29:538-543. PMID:
19141364.


11. Ockene IS, Miller NH. American Heart Association Task Force on Risk Reduction. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. Circulation 1997;96:3243-3247. PMID:
9386200.


12. Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989;298:789-794. PMID:
2496858.



13. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86-97. PMID:
12837716.


14. Enstrom JE, Heath CW Jr. Smoking cessation and mortality trends among 118,000 Californians, 1960-1997. Epidemiology 1999;10:500-512. PMID:
10468422.


15. Molyneux A. Nicotine replacement therapy. BMJ 2004;328:454-456. PMID:
14976103.



16. Fiore MC, Novotny TE, Pierce JP, Giovino GA, Hatziandreu EJ, Newcomb PA, et al. Methods used to quit smoking in the United States. Do cessation programs help? JAMA 1990;263:2760-2765. PMID:
2271019.


17. Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 2004;5:55-65. PMID:
14708004.



18. Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther 2009;31:463-491. PMID:
19393839.


19. Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55. PMID:
16820546.


20. Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63. PMID:
16820547.


21. Tonstad S, T├Ėnnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296:64-71. PMID:
16820548.


22. Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology 2009;14:384-392. PMID:
19192221.


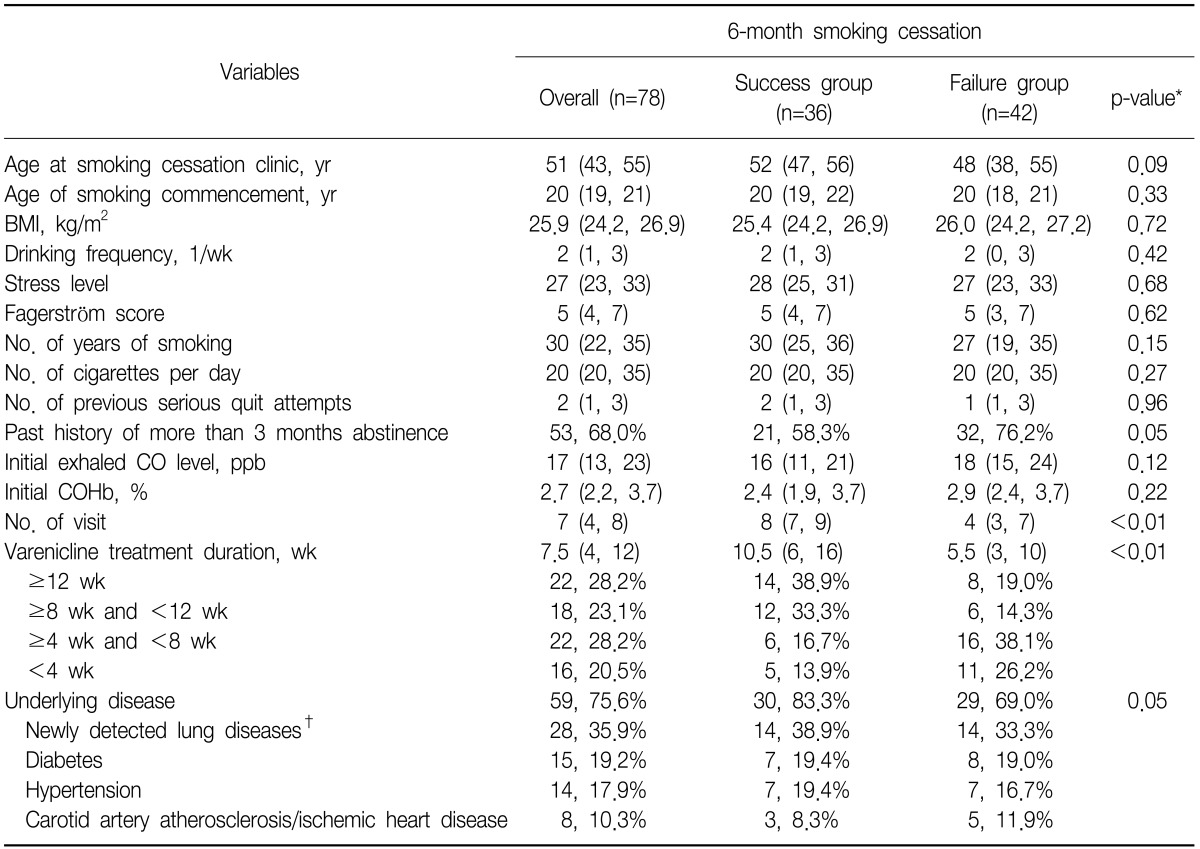
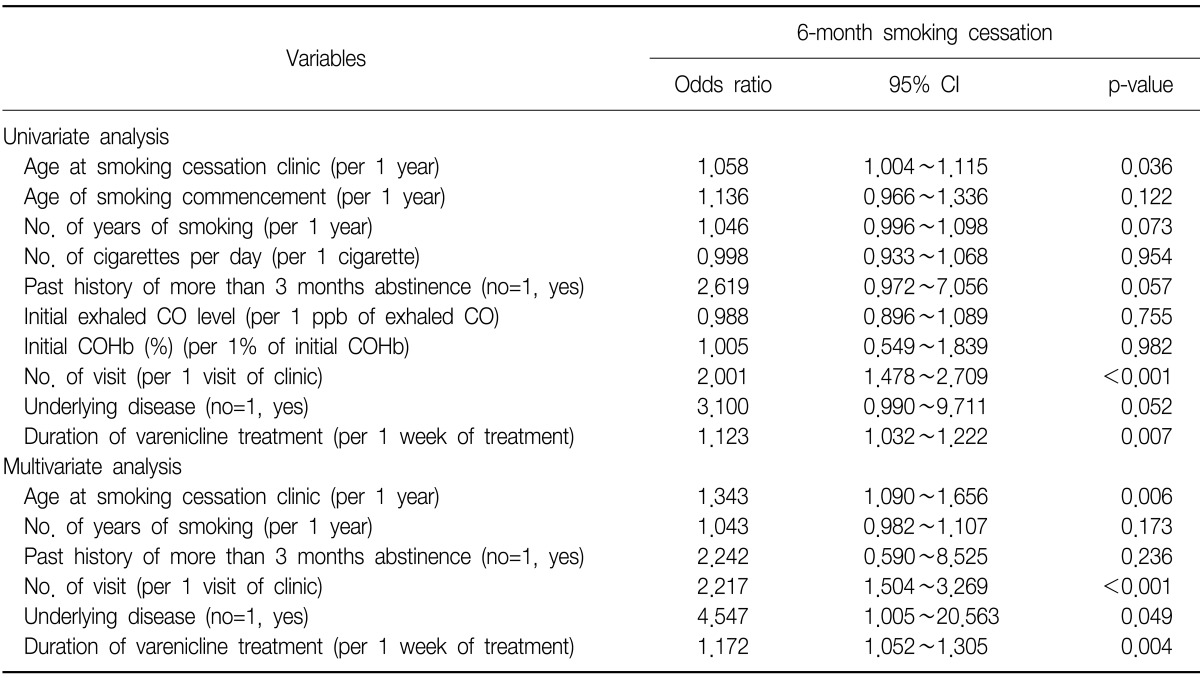
23. Song B, Yun WS, Choi EY, Cheong YS, Park EW. Smoking cessation rate and related factors of varenicline in clinical practice. Korean J Fam Med 2011;32:112-119.






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




