 |
 |
| Tuberc Respir Dis > Volume 73(1); 2012 > Article |
|
Abstract
Background
This study evaluates the bacterial pathogens of Ventilator-associated pneumonia (VAP) in a tertiary referral hospital.
Methods
A total of 109 bacterial pathogens from 91 adult patients with VAP, who were admitted to the medical intensive care unit from January 2008 to December 2009, were examined. Clinical characteristics, bacterial pathogens, and resistance profiles were analyzed.
Results
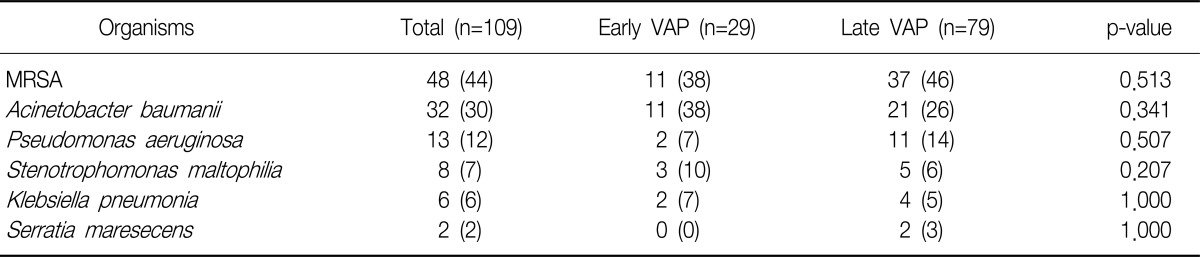
Staphylococcus aureus (44%) was the most frequently isolated. Acinetobacter baumanii (30%), Pseudomonas aeruginosa (12%), Stenotrophomonas maltophilia (7%), Klebsiella pneumoniae (6%), and Serratia marcescens (2%) were isolated from the transtracheal aspirates or bronchoalveolar lavage in patients with VAP. There was no significant difference of bacterial pathogens between early and late onset VAP. All isolated S. aureus were methicillin resistant S. aureus; the imipenem resistance rate of A. baumanii was 69%.
Ventilator associated pneumonia (VAP) occurs 48 hours after intubation and mechanical ventilation. It is a common infectious disease that is found in intensive care unit (ICU), which occurs in 8~38% of patients who underwent mechanical ventilation1. The incidence of pneumonia has been known to be higher in ICU patients than in general ward patients, and even 3~10-fold higher in patients who underwent mechanical ventilation2-9. In addition, the mortality of VAP has been reported to be 24~76%, which is higher than those of other hospital acquired infections10.
Common causative pathogens of VAP include Gramnegative bacteria such as Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter species, and Gram-positive bacteria such as Staphylococcus aureus9-14. As the type of causative pathogens and the rate of drug-resistant pathogens may vary depending on region and hospital, data of microbial surveillance are required for each region. As most studies on causative pathogen, however, have been conducted in western counties, domestic data are insufficient. Accordingly, this study was conducted to investigate the bacterial pathogens of VAP in a domestic tertiary referral hospital.
This study was conducted on 104 patients who satisfied the diagnostic criteria of VAP via the retrospective analysis of their medical records and diagnostic results among 713 patients who underwent intubation and mechanical ventilation in the ICU of the department of internal medicine, Chonnam National University Hospital from January 1st, 2008 to December 31st, 2009. Thirteen patients who had unknown causative pathogens were excluded among the 104 patients.
Pneumonia was diagnosed by chest radiograph and clinical and laboratory findings. If the patients, who had a new pulmonary infiltration, satisfied 2 or more conditions among the cases of body temperature 38.3Ōäā or higher, purulent bronchial secretions, and a leukopenia or leukocytosis (<4,000 or >11,000 mm3), they were diagnosed with pneumonia15. The patient's age, gender, a history of antibiotic therapy before hospitalization, a history of hospitalization in other hospitals, concurrent diseases, causes of mechanical ventilation, causative pathogens, antibiotic resistances, and mortality were retrospectively examined. The subjects were divided into two groups according to the development time of VAP: early VAP group where VAP occurred within 5 days after mechanical ventilation and late VAP group where VAP occurred 5 days or later after mechanical ventilation. A comparative analysis was conducted between the two groups10,15.
Specimens were collected from all patients with the suspected pneumonia via transtracheal aspirate (TTA) or bronchoalveolar lavage (BAL) before antibiotic administration, followed by qualitative or quantitative culture. In the cases of deterioration via a chest radiograph, persistent fever, or worsening of PaO2/FiO2 rate even after antibiotic administration, antibiotics were changed. Specimens were again collected from the patients via TTA or BAL before antibiotic change, followed by culturing. VITEK device (Vitek-120; Biomerieux, St. Louis, MO, USA) was used for culturing, and antibiotic susceptibility test was conducted in accordance with the National Committee for Clinical Laboratory Standard (NCCLS)16.
The isolated bacteria were classified into causative pathogens according to the followings17: 1) definite pathogen: if bacteria from the blood culture and those from TTA or BAL specimens are identical; 2) probable pathogen: if qualitative culture has 105 colony-forming unit (CFU)/mL or more in TTA specimens or 104 CFU/mL or more in BAL specimens; 3) possible pathogen: if bacteria are cultured from the TTA specimen via a qualitative culturing method.
Median and interquartile range (IQR) were used for continuous variables, whereas frequency and percentile were used for the remaining variables. Pearson's Chi-square test or Fisher's exact test was conducted for patient's age, concurrent diseases, causes of mechanical ventilation, and a history of antibiotic medication in preceding 90 days, a history of hospitalization, and causative pathogens of VAP. Mann-Whitney U test was conducted for the patient's age and interval from mechanical ventilation to the development of VAP. If p<0.05, it was considered statistically significant.
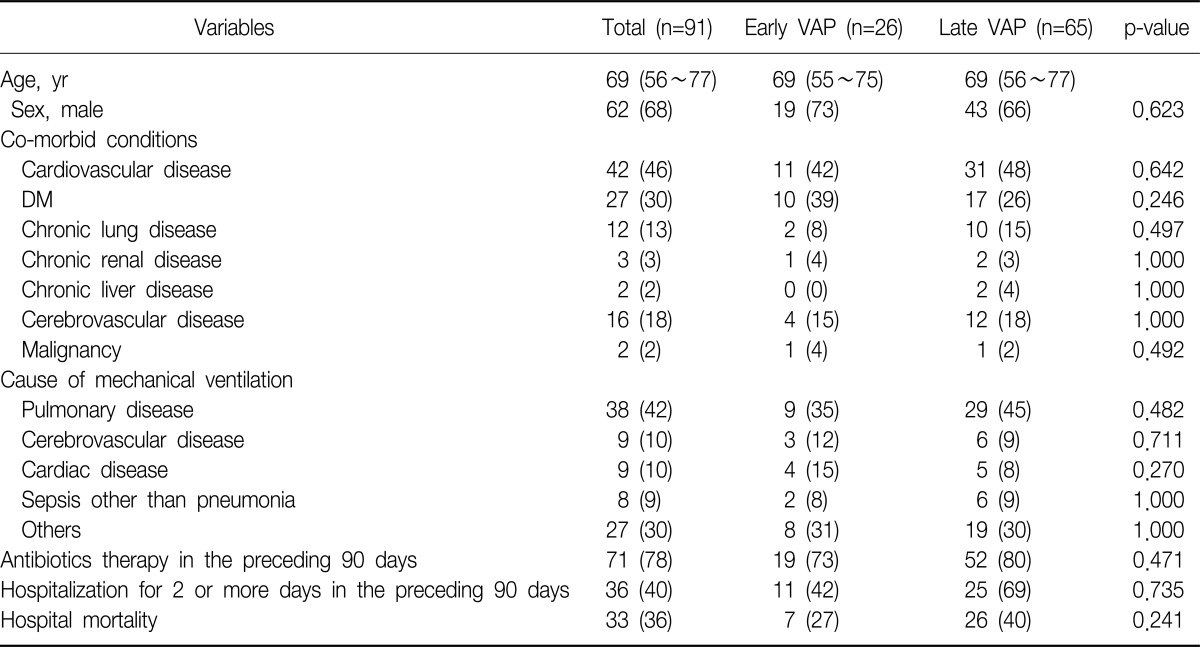
The median age of the patients was 69 years (IQR, 56~77 years). The male patients accounted for 68% of the total subjects. VAP occurred median 7 days (IQR, 5~13 days) after mechanical ventilation. Pulmonary diseases were the most common cause of mechanical ventilation, which accounted for 42%. The in-hospital mortality was 40%. When the patients were divided into the early and late VAP groups according to the development time of VAP, no difference in the patient's age, gender, underlying diseases, causes of mechanical ventilation, a history of antibiotic medication and hospitalization in preceding 90 days, and mortality was found between the two groups. The time interval from mechanical ventilation to the occurrence of VAP was shown to be median 4 days (IQR, 4~5 days) and median 10 days (IQR, 7~15 days) in the early and late VAP groups, respectively (Table 1).
A total of 109 bacteria were identified from 91 patients. Two or more bacteria were identified from 18 patients, and no difference was found between the early and late VAP groups (3/26 vs. 15/65, p=0.257). Among the 18 patients who had two or more bacteria, the different bacteria were cultured simultaneously in 12 patients (67%), and time-dependent changes were shown in six patients (33%). No difference was found between the early and late VAP groups (p=1.000). As for the changes in bacterial identification according to development time, S. aureus was first identified, and subsequently A. baumanii was identified in three patients. S. aureus, and subsequently Stenotrophomonas maltophilia, A. baumanii and subsequently P. aeruginosa, K. pneumoniae and subsequently P. aeruginosa were identified in one patient, respectively.
The most commonly identified bacteria was shown to be S. aureus, which accounted for 44%. A. baumanii, P. aeruginosa, S. maltophilia, K. pneumoniae, and Serratia marcescens followed S. aureus in that order. However, no difference in the frequency of the identified bacteria was found according to the onset time of VAP (Table 2).
One hundred nine causative pathogens were divided into the definite, probable, and possible pathogens: 5 (19%), 0 (0%), and 21 (81%), respectively, for the early VAP group, and 7 (11%), 11 (17%), 47 (72%), respectively, for the late VAP group. There were no difference between the late VAP group and the early VAP group (p=0.061). Among 12 cases of definite pathogen, S. aureus (6 cases, 50%) was the most commonly found, and A. baumanii, and P. aeruginosa were identified in two cases, respectively, and K. pneumoniae, and S. maltophilia were shown in identified in one case, respectively.
As for the drug-resistant pathogens among the isolated bacteria, all S. aureus belonged to methicillin resistance S. aureus (MRSA). Sixty nine percent (22/32) of A. baumanii was imipenem-resistant. No difference in the imipenem-resistant A. baumanii was found between the early and late VAP groups (73% [8/11] vs. 67% [14/21], p=1.000). K. pneumoniae was shown to have an extended spectrum beta-lactamase (ESBL) positivity rate of 67% (4/6). Two pathogens identified from the early VAP group were all negative for ESBL, whereas four pathogens identified from the late VAP group were all positive for ESBL.
In this study, S. aureus was shown to be the most common causative pathogen of VAP at ICU, and A. baumanii, P. aeruginosa, S. maltophilia, K. pneumonia, and Serratia marcescens followed S. aureus in that order. No difference in the causative pathogens was found between the early and late VAP groups according to the development time of VAP.
VAP is one of serious complications that occur at ICU. As its causative pathogens are antibiotic resistant in many cases, it is difficult to select appropriate antibiotics. In addition, the mortality has been reported to increase if an early antibiotic treatment is not provided to patients with VAP18. Thus, before the identification of causative pathogens, antibiotic selection is performed to target causative pathogens mainly identified in the corresponding region. However, most studies on causative pathogens have been conducted in western countries, and few studies have been conducted in Korea. According to the SENTRY antimicrobial surveillance program operated in US, Europe, and South America, P. aeruginosa (27%) is the most common causative pathogen taken all regions together, and S. aureus (20%), and Acinetobacter species (14%) follow P. aeruginosa in that order. In the US, S. aureus (32%) is the most common causative pathogen, followed by P. aeruginosa (21%), Enterobacter species (9%), and Acinetobacter species (4.4%) in that order11. Meanwhile, according to a recent study on the causative pathogens of nosocomial pneumonia in Asia, S. aureus (27%) was the most common causative pathogen of nosocomial pneumonia in Korea, and Acinetobacter species (16%), P. aeruginosa (14%), and K. pneumoniae (9%) followed S. aureus in that order17. Although this was different from the result of foreign studies, it was similar to the result of this study. Thus, in Korea, causative pathogens of VAP are likely to occur at the aforementioned frequency.
It is uneasy to conduct the microbiological diagnose of VAP. A quantitative culture test of lower airway specimens via bronchoscopy has been reported to have high sensitivity and specificity19. However, as bronchoscopy has not been conducted in all patients in this study, the pathogens, which were identified according to the method of the recent study on the causative pathogen of nosocomial pneumonia in Asia, were divided into the definite, probable, and possible pathogens, followed by comparative analysis to investigate their role as causative pathogens of VAP17. In the case of the definite pathogens with the highest possibility of being causative pathogens, S. aureus was the most common causative pathogen, and A. baumanii and P. aeruginosa followed S. aureus in that order, which was similar to the overall result. Thus, these pathogens are likely to be the main causative pathogens of VAP.
Causative pathogens of VAP has been known to vary depending on the development time of VAP. In the case of early VAP that occurs within 5 days after mechanical ventilation following intubation, antibiotic sensitive bacteria such as S. aureus, Streptococcus pneumoniae, and Haemophilus influenzae are main causative pathogens. Meanwhile, in the case of late VAP that occur 5 days or later after mechanical ventilation following intubation, multidrug-resistant bacteria such as P. aeruginosa, A. baumanii, and MRSA are main causative pathogens10,15. In this study, no difference in the causative pathogens was found between the early and late VAP groups. This was inconsistent with the results of previous studies, and might be associated with a local increase in drug-resistant bacteria. The recent study on nosocomial pneumonia in Asia including Korea also reported that Acinetobacter species, P. aeruginosa, S. aureus, and K. pneumoniae were the most common pathogens identified from both early and late nosocomial pneumonia17. Thus, it could be possible that VAP that occur in Korea are antibiotic resistant pneumonia although they belong to early VAP. However, the result of this study might be attributable to the fact that most patients of this study had a previous history of antibiotic medication (78%) and some had of recent hospitalization (40%). Thus, drug-resistant bacteria might have been identified from the early VAP group.
The recent study on the causative pathogens of nosocomial pneumonia in Asia reported that A. baumanii, the second commonest causative pathogen of VAP in this study, more commonly occurred compare to US or Europe, and that it was resistant to many antibiotics11,17. In particular, the imipenem-resistance rate of Acinetobacter species was more than 80% in Thailand, Malaysia, and India, and 28% in Korea. Although the overall imipenem-resistance rate was reported to be high in Asia, the rate was relatively low in Korea. However, this rate could not represent general imipenem-resistance rate of Acinetobacter species in Korea due to only a small specimen number of 29 Korean cases in this study17. The Korean Nationwide Surveillance of Antimicrobial Resistance (KONSAR) was shown to have the largest subject number. According to the KONSAR where drug susceptibility test was conducted in 24 hospitals, the imipenem-resistance rate of A. baumanii was reported to be 51%20, and the carbapenem-resistance rate of A. baumanii identified in the ICU of a university hospital was reported to be 53% (26/49)21. In this study, the imipenem-resistance rate was shown to be 69%. Thus, the imipenem-resistance rate of A. baumanii is expected to be high in Korea.
VAP is a fatal disease with a high mortality. The causative pathogens of VAP may vary depending on country, region, and hospital. If information on the causative pathogens of VAP is available, it could increase the possibility of appropriate antibiotic therapy, thereby reducing the mortality and improving the prognosis. In summary, this study was conducted to investigate the causative pathogens of VAP in a tertiary referral hospital. As a result, S. aureus and A. baumanii were shown to be the most common and second commonest causative pathogens of VAP, respectively.
Acknowledgements
This study was supported by a grant (CRI11004-1) of the Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 2005;33:2184-2193. PMID: 16215368.


2. Bell RC, Coalson JJ, Smith JD, Johanson WG Jr. Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med 1983;99:293-298. PMID: 6614678.


3. Celis R, Torres A, Gatell JM, Almela M, Rodr├Łguez-Roisin R, Agust├Ł-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest 1988;93:318-324. PMID: 3338299.


4. Chevret S, Hemmer M, Carlet J, Langer M. European Cooperative Group on Nosocomial Pneumonia. Incidence and risk factors of pneumonia acquired in intensive care units: results from a multicenter prospective study on 996 patients. Intensive Care Med 1993;19:256-264. PMID: 8408934.



5. Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect 1996;11:32-53. PMID: 8885061.

6. Cross AS, Roup B. Role of respiratory assistance devices in endemic nosocomial pneumonia. Am J Med 1981;70:681-685. PMID: 6938128.


7. Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation: prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 1989;139:877-884. PMID: 2930067.


8. Langer M, Mosconi P, Cigada M, Mandelli M. Intensive Care Unit Group of Infection Control. Long-term respiratory support and risk of pneumonia in critically ill patients. Am Rev Respir Dis 1989;140:302-305. PMID: 2764365.


9. Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. EPIC International Advisory Committee. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA 1995;274:639-644. PMID: 7637145.


10. Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903. PMID: 11934711.


11. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010;51(Suppl 1):S81-S87. PMID: 20597676.



12. Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000;21:510-515. PMID: 10968716.


13. Alc├│n A, F├Ābregas N, Torres A. Hospital-acquired pneumonia: etiologic considerations. Infect Dis Clin North Am 2003;17:679-695. PMID: 15008591.


14. Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of healthcare-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005;128:3854-3862. PMID: 16354854.


16. Clinical and Laboratory Standards Institute. Document M100-S20. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement. 2010. Wayne, PA: Clinical and Laboratory Standards Institute.
17. Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 2011;184:1409-1417. PMID: 21920919.


18. Luna CM, Aruj P, Niederman MS, Garz├│n J, Violi D, Prignoni A, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J 2006;27:158-164. PMID: 16387949.


19. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. PMID: 15699079.


20. Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, et al. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J 2011;52:793-802. PMID: 21786445.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




