 |
 |
| Tuberc Respir Dis > Volume 86(2); 2023 > Article |
|
Abstract
Chronic obstructive pulmonary disease (COPD) affects close to 400 million people worldwide. COPD is characterized by significant airflow limitation on spirometry. Most patients with COPD are diagnosed in their fifth or sixth decades of life. However, the disease begins much earlier. By the time airflow limitation is detected on spirometry, patients with COPD have lost close to 50% of their small airways. Thus, identification of patients with early COPD, defined as persons with preserved spirometry, who demonstrate pathologic or functional hallmarks of COPD, is essential for disease modification and ultimately disease elimination. This paper provides an up-to-date overview of the current case definition of early COPD, its importance, the novel technologies required for its detection in young adults and future directions in therapeutics for treatment.
There are currently 392 million persons around the world who are living with chronic obstructive pulmonary disease (COPD); 81% of these patients reside in low or middle income countries (LMICs) [1]. In Korea, approximately 3.8 million residents between 30 and 79 years of age have COPD, representing 11.4% of the adult population [1]. Although cigarette smoking is the leading risk factor for COPD, according to the Global Burden of Disease (GBD) 2019, approximately 50% of the risk is at least in part related to air pollution and occupational exposures with the attributable risk amplifying in those living in LMICs [2]. In life-time non-smokers, air pollution is the leading known risk factor for COPD [3].
COPD is characterized by persistent airflow limitation that is incompletely reversible with bronchodilators [4]. Although the traditional paradigm is that rapid decline in lung function is the main driver of airflow limitation in COPD, recent data suggest that there are multiple different trajectories of lung function that lead to COPD [5]. Of these, the most pernicious (and most common) is poor lung growth and development, which can occur anytime from in utero to young adulthood [5]. Currently, aside from smoking cessation for active smokers, there are no disease modifying therapies for COPD [4]. One reason for this is that COPD is typically diagnosed in persons with a smoking history in their fifth to sixth decades of life (or older) and by this time, their disease is “fixed” and cannot be modified [4]. Thus, for disease modification, patients must be identified earlier in their disease course, perhaps in their third or even second decades of life, when they have only a modest amount of symptoms [4]. However, 20- or 30-year olds rarely present to health professionals and even when they do, spirometry is relatively too crude and insensitive to detect the significant hallmarks of COPD in young adults. Reliable and consistent diagnosis of “early” COPD is essential for disease modification and to enable elimination of COPD in future generations [4]. In this review, we will review the current case definition of early COPD, its importance, the novel technologies required for its detection in young adults and future directions in therapeutics for disease modification and ultimately disease elimination.
Using search terms “COPD,” “early,” “young,” and “chronic obstructive pulmonary disease,” PubMed was interrogated to February 13, 2023 to identify relevant papers on this topic. In addition, the reference lists of review papers and published systematic reviews were searched for relevant articles that may have been missed during the electronic search.
For this review, we will use the Global initiative for chronic Obstructive Lung Disease (GOLD)’s definition of COPD, which is based on forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of less than 0.70 in the presence of chronic respiratory symptoms such as dyspnea, cough, or sputum production [6]. “Early COPD” is defined as COPD (with or without significant airflow limitation) in adults typically less than 50 years of age, who have pathological or functional hallmarks of COPD [7]. Some have used the term “young COPD” in reference to COPD in persons less than 50 years of age and reserved the term “early COPD” to indicate the biologically early timepoints in COPD pathogenesis [8]. As the pathogenesis of COPD can begin at any age, early COPD can, in theory, be diagnosed in persons over 50 years of age. Moreover, whereas spirometry is sufficient in making the diagnosis of COPD in patients less than 50 years of age, for diagnosis of early COPD, additional testing is frequently required. In this paper, we will focus on early rather than young COPD (Table 1).
As noted previously, COPD is defined on the basis of reduced lung function (i.e., airflow limitation that cannot be fully reversed) [6]. Because alterations in lung function can occur at any time from conception to death, COPD must be viewed across the full spectrum of life. Lung function trajectory broadly follows three phases over time (Figure 1): (1) lung growth and development, (2) lung maintenance, and (3) age-related lung function loss [4]. The first phase, lung growth and development, occurs from the womb to early adulthood (approximately 20 to 25 years of age). The second phase, lung maintenance, is predominant between approximately 25 and 35 years of age and the third phase is generally observed beyond 35 years of age [4]. Thus, the lung function in a given older person reflects an integrated signal from all three phases of life. A significant reduction in lung function in adults may be the consequence of perturbations in any of these phases of life. In high-income countries, approximately two-thirds of the COPD cases are accounted for by impaired lung growth and development (phase 1 abnormality); and rapid decline in lung function reflective of either phase 2 or phase 3 disturbances is responsible for the remaining one-third of the cases (Figure 1) [5].
The most important risk factors for poor lung growth and development include prematurity, bronchopulmonary dysplasia, childhood asthma, and pneumonias as well as childhood environmental exposures to toxins and pollutants such as second hand cigarette smoke and air pollution particles [4]. This is true even in high-income countries. For example, a study from the United Kingdom showed that chronic exposure to small particulate matter (less than 10 μm in mean aerodynamic diameter) was inversely related to the lung function of children 8 to 12 years of age; interestingly, this relationship was largely explained by the presence of black carbon particles in airway macrophages of these children [9]. While the pathophysiology of pollution-related COPD is unknown, there is growing evidence that small particles drive a low-grade inflammatory process (mediated by macrophages) in the small airways leading to bronchiolitis [10].
The most important risk factor for dysfunctional lung maintenance (phase 2) is cigarette smoking (though other forms of smoking such as cannabis and e-cigarettes are emerging risk factors) [11,12]. In LMICs, both indoor and outdoor air pollution is the leading risk factor for COPD especially among women [2]. Interestingly, once these particles are inhaled into the airways, they are taken up by lung macrophages and remain inside these cells for decades [13]. Particle-laden macrophages, in turn, lose some of their phagocytic ability resulting in impaired lung maintenance and predisposing the host to recurrent respiratory tract infections [14]. For phase 3, cigarette smoking is the leading risk factor in high-income countries such as Korea, increasing the age-related decline by 100% compared with non-smokers [15].
It is now well established that a majority of small airways (defined as airways less than 2 mm in diameter) are lost by the time fixed airflow limitation is observed on spirometry [16]. In a landmark study, Koo et al. [16] used micro-computed tomography (micro-CT) to carefully measure terminal and transitional bronchioles in lung samples from patients with mild (GOLD 1; FEV1 ≥80% of predicted), moderate (GOLD 2; FEV1 50% to 79% of predicted), and very severe COPD (GOLD 4; FEV1 less than 30% of predicted) as well as those from smokers without COPD. They found that compared with control smokers, GOLD 1 COPD samples contained 40% fewer terminal bronchioles and 56% fewer transitional bronchioles; these numbers increased to 43% and 59%, respectively, in GOLD 2 and 68% and 90%, respectively, in GOLD 4 COPD samples. Importantly, among terminal bronchioles that could be observed on micro-CTs, 41% of the airways were abnormal (either thickened or obstructed) in GOLD 1 samples, 37% were abnormal in GOLD 2 samples, and 77% were abnormal in GOLD 4 samples. In contrast, only 12% of the terminal bronchioles in the smoking control samples were abnormal by micro-CT. On parenchymal assessment, the authors found there was a 33% loss of alveolar surface area in GOLD 1 samples; 45% loss in GOLD 2 samples; and 79% loss in GOLD 4 samples [16]. Together, these data indicate that by the time patients are diagnosed with even mild COPD (GOLD 1), they have lost approximately 50% of their small airways and a third of their gas-exchanging alveolar surface area in their lungs. As lung regeneration is not possible at this point, it is crucial to identify “COPD” patients before they develop airflow limitation on spirometry and intervene at this early stages of disease (more on this later) [4]. As the pathologic process of COPD begins in small airways (i.e., airways less than 2 mm in diameter) [17], the diagnostic technologies must be able to accurately and precisely detect abnormalities in these airways, even when large airways are physiologically and anatomically normal.
Spirometry is the gold standard in the diagnosis of COPD [6]. On spirometry, obstruction is characterized by airflow limitation, which manifests as reduced FEV1, a reduced FEV1/FVC ratio, a concave flow-volume loop or all of the above (Table 2). However, as noted previously, by the time patients are diagnosed with even mild (GOLD 1) COPD (normal FEV1 but reduced FEV1/FVC ratio), they have lost nearly half of their small airways and a third of their gas-exchanging units in lungs [16]. This is because the most frequently used spirometric values (e.g., FEV1 and FVC) generally reflect airflow in medium to large sized airways and not the small airways, which are the sites of disease in COPD (Table 2). In contrast, the forced mid-expiratory flow (FEF25%-75%), also known as maximum mid-expiratory flow on spirometry, is thought to reflect airflow in small to medium sized airways. One study found that low FEF25%-75% in the presence of normal FEV1 and FVC values was associated with increased future occurrence of COPD, elevating incidence by 3- to 4-fold [18]. Because FEF25%-75% values are typically concordant with FEV1 and in particular FVC values, FEF25%-75% often has to be corrected for patient’s FVC. One major diagnostic limitation of the FEF25%-75% value is its high noise-to-signal ratio [19]. Thus, FEF25%-75% is rarely useful in clinical decision making or diagnosis [19]. Similar issues have also plagued FEF50%, which measures forced expiratory flow at 50% of FVC, another parameter that reflects small to medium sized airways.
Another popular method for detecting small airway disease is impulse oscillometry, which is a variant of forced oscillation technique that was first described over 50 years ago [20]. Unlike spirometry, which requires patient cooperation for the FVC maneuver, impulse oscillometry is performed while the patient is spontaneously breathing tidal volume. Thus, active patient cooperation is not required. The reader is referred elsewhere for a more detailed description of impulse oscillometry [20,21]. In brief, sound waves are generated and dispersed into the airways. Lower frequency waves are able to penetrate deep into the lungs; whereas higher frequency waves are filtered out in the larger airways. In general, sound frequency from 4 to 30 Hz is used to calculate respiratory impedance (which is the ratio of pressure changes relative to flow changes) at these frequencies. The respiratory impedance includes two important measures: an in-phase component (or resistance) and an out-of-phase component (reactance). The resistance reflects loss in energy of the sound waves as they traverse the airways; whereas reactance reflects energy storage in the lungs. In the largest study of its kind to date, Crim et al. [22] showed that patients with COPD (n=2,054) demonstrate significantly higher resistance at all lower frequencies (between 5 and 20 Hz) and reduced reactance at 5 Hz compared with non-smoking or smoking controls. On the other hand, respiratory impedance parameters are similar between smoking and non-smoking controls. Jetmalani et al. [23] evaluated 80 smokers with preserved spirometry and showed that those who were wheezy had significantly higher resistance values at lower frequencies compared with smokers who were asymptomatic. A major limitation of this and other studies was their cross-sectional methodology, which reduced the strength of causal inferences that could be made from the study results.
One of the largest longitudinal data of impulse oscillometry are found in the Assessment of Small Airways Involvement in Asthma (ATLANTIS) Study, which evaluated the performance of impulse oscillometry and other technologies in detecting small airways disease in adults with asthma (n=773). The investigators found that airway resistance values at lower frequencies were four times higher in patients with asthma than in healthy controls without asthma. Similarly, reactance at low frequencies was three times higher in patients with asthma than in healthy control subjects [24]. Importantly, the impulse oscillometry measurements were most predictive of small airway dysfunction in these patients and a composite ordinal score (consisting of airway resistance and reactance values at low frequencies) was significantly associated with patients’ health status and their future risk of exacerbations [25]. Together these data from patients with asthma and COPD suggest that small airway dysfunction can be detected by impulse oscillometry and is predictive of poor health status and future occurrence of exacerbations in patients with established airways disease. However, whether these measurements can predict which persons will go onto develop COPD in the future is not known.
Another novel method for detecting small airways disease is single or multiple breath nitrogen washout test, which have been described in detail previously [26]. In brief, the basic principle of this test is to determine the homogeneity (or heterogeneity) of ventilation across the lungs by measuring the lung’s ability to release a tracer gas during expiration. In general, the longer it takes to fully washout the tracer gas, the greater the ventilation inhomogeneity there is across the lungs, indicating small airways disease. By calculating different slopes on the multiple breath washout test, the source of the ventilation inhomogeneity can also be determined: ventilation heterogeneity arising from large conducting airways versus that arising from the smaller, distal airways. The lung clearance index (LCI) is currently the most frequently used washout technique in clinical practice. LCI is calculated based on the cumulative expired volume of air exhaled during the washout portion of the test, adjusted for the person’s functional residual capacity (FRC). Thus, the readout for LCI is the number of FRCs required to fully clear the tracer gas.
In the Study of Men Born in 1913 and 1923, Olofson et al. [27] evaluated 625 men aged 50 to 60 years in 1973 with spirometry, respiratory questionnaires and a single breath nitrogen washout test. Ventilation inhomogeneity as measured by the nitrogen slope during the baseline washout test was more strongly associated with future occurrence of COPD (based on a physician diagnosis) or COPD hospitalization compared with traditional spirometric values such as baseline FEV1 [27]. This also applied to the endpoints of all-cause mortality or rate of FEV1 decline over time in this cohort [28,29]. These data have been generally replicated in other studies [30,31]. In a study of two large community cohorts, Buist et al. [31] found that 80% to 90% of smokers with preserved spirometry, who demonstrated an abnormal N2 washout test at baseline, developed significant airflow limitation over an approximate 10-year span, suggesting that the washout test can be used as a “screening” method to identify smokers at high risk of developing COPD in the future. In aggregate, these data highlight nitrogen washout tests as a promising technology in identifying persons with small airway dysfunction, who are likely to develop clinically significant COPD in the future.
Multistage cardiopulmonary exercise testing can also reveal small airway dysfunction in persons who have normal spirometry. In general, compared to non-smoking control subjects, smokers at risk for COPD have reduced exercise capacity and increased total lung resistance, and in particular increased inspiratory resistive work of breathing, even when they demonstrate normal airflows and lung volumes [32].
Over the past decade, there has been a growing interest in using imaging tools to identify persons with small airways disease [33]. To date CT scanning has been the best studied. Previous studies have shown that some smokers with preserved spirometry demonstrate emphysematous changes on CT images [33,34]. These patients often also have reduced diffusion capacity of lungs for carbon monoxide (DLCO) or transfer coefficient. In smokers with preserved spirometry, the presence of emphysema on CT scans is associated with more rapid decline in FEV1 over time and increased risk of significant airflow limitation [34]. Similarly, an elevated residual volume to total lung capacity ratio based on lung plethysmography or CT data and reduced total airway count on CT are also associated with more rapid decline in lung function and increased risk of COPD over time [35,36]. However, despite the statistical significance of these associations, their performance to predict (or discriminate) smokers who will progress to COPD from those who will not is relatively poor. Thus, none of these methods are ready for clinical implementation. A more promising method is to apply machine learning (e.g., deep learning) algorithms on clinical CT scans (for those done for lung cancer screening) to enhance prediction. Using deep learning methods on CT scans, Tang et al. [37] showed that performance of CT data (to discriminate COPD from healthy smokers on these images) can be significantly improved. In this study, the area under the curve (AUC) was approximately 0.85 [37]. In contrast, the use of densitometry measures to estimate the burden of emphysema on CT scans had an AUC of approximately 0.7 [37].
While the small airways are beyond the resolution of most clinical CT scanners, co-registration of inspiratory and expiratory scans on a voxel-by-voxel basis reveals differential regions of gas emptying, which can be quantified using novel computerized algorithms such as parametric response mapping (PRM) [38]. Areas containing diseased small airways, in general, demonstrate lower and more variable gas emptying rates owing to differential time-constants, while non-diseased areas empty faster and in a more homogenous fashion. Thus, in diseased lungs, PRM values are more heterogeneous compared with normal healthy lungs.
An emerging technology of interest is pulmonary magnetic resonance imaging (MRI) with inhaled hyperpolarized xenon (129Xe) or helium (3He). Pulmonary MRI does not emit X-ray radiation and thus it is safe to use repeatedly in all populations including children and expectant mothers. With the restricted worldwide availability of helium, xenon is now the preferred inert gas of choice for pulmonary MRI. Xenon has the added advantage that it is diffusible across tissue and soluble in blood, enabling quantification of regional perfusion and gas uptake. Recent studies suggest that this technology can detect subtle but important differences in the small airways between healthy smokers and non-smokers with the former demonstrating thicker alveolar walls [39]. Pulmonary MRI measurements may also have predictive value in identifying smokers who are likely to progress to COPD from those who will not [40]. However, large longitudinal studies of hyperpolarized gas MRI are needed to better understand the role of pulmonary MRI as a predictive tool in identifying smokers who are at high risk of disease progression (Table 2).
As noted previously, most current or former smokers do not develop significant airflow limitation until they are in their 50’s or 60’s of age. As such, COPD diagnosis in young adults based on spirometric criteria is extremely challenging. However, despite having normal spirometric values, many smokers with preserved spirometry are symptomatic and experience frequent exacerbations (Figure 2). In the largest study of its kind, Woodruff et al. [41] evaluated 849 current or former smokers (with >20 pack-years of smoking history) who had normal spirometry at baseline in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). They found that approximately 50% of the smokers in this study were symptomatic based on the COPD Assessment Test (CAT) score of 10 or greater. Interestingly, the symptomatic smokers’ group (with preserved spirometry) was more likely to be females, current smokers, and non-whites, who had a history of asthma or COPD compared to nonsymptomatic smokers with preserved spirometry. Most importantly, over a median follow-up of almost 3 years, the rate of exacerbation, defined as a respiratory event requiring the use of antibiotics or prednisone, hospitalization or emergency department visit, was 3.3 times higher in symptomatic smokers than that of nonsymptomatic smokers (0.27 events per year vs 0.08 events per year, p<0.001). Moreover, the symptomatic smokers were able to walk, on average, 50 m less on the 6-minute walk test than nonsymptomatic smokers with preserved spirometry (Figure 2).
Patients typically present to health professionals because of ongoing symptoms. In COPD, the most common symptoms are exertional dyspnea, persistent cough, and sputum production. Based on the assumption that a majority of symptomatic smokers will eventually develop COPD [42], many in clinical practice are treating symptomatic smokers with preserved spirometry with anti-COPD therapies such as inhaled corticosteroids (ICS) or long acting bronchodilators [41]. A recent randomized controlled trial of symptomatic smokers with preserved spirometry, Redefining Therapy in Early COPD (RETHINC), showed that a 12-week treatment of a long acting beta-2 agonist/long acting muscarinic antagonist (LABA/LAMA) combination did not improve patients’ symptoms or quality of life [43]. The LABA/LAMA combination improved FEV1 in this population by approximately 40 mL, which is lower than approximately 200 mL improvement, which has been observed in patients with GOLD 2 or 3 COPD [44]. Thus, smokers with preserved spirometry should not be empirically treated with long acting bronchodilators. Moreover, these data suggest that symptoms alone are too insensitive for use as diagnostic indicators of “early” COPD.
It should also be noted that the “placebo effect” is very strong in symptomatic smokers with preserved spirometry. In the RETHINC trial, for example, the average improvement in the St. George’s Respiratory Questionnaire (SGRQ) score and the CAT score over 12 weeks was 8.9 and 4.5 points, respectively, in the placebo group [43]. As the minimal clinically important difference for SGRQ is 4 points and that for CAT is 2 points, a majority of symptomatic smokers in the placebo group felt significantly better at 3 months of follow-up even without any treatment than they did at baseline. Thus, future trials evaluating new approaches or novel therapies for symptomatic smokers with preserved spirometry should carefully consider the strong “placebo” effect in their design [44]. Moreover, in the RETHINC trial, the predominant symptoms in smokers with preserved spirometry were cough and sputum production (i.e., “smokers’ cough”) rather than exertional dyspnea with 70% reporting a history of chronic bronchitis (based on the CAT) [43]. This may have important implications in the choice of therapeutics for future evaluation.
Another approach is to first identify symptomatic (current or formers) smokers, who in addition to symptoms, have physiological or anatomical hallmarks of COPD such as rapid decline in FEV1 (>40 mL/year), abnormal LCI or impulse oscillometry results, pulmonary function test (PFT) evidence of gas trapping or decreased DLCO, or CT evidence of emphysema [4] and then treat these individuals with current anti-COPD therapies. The GOLD committee has labelled these persons as having “pre-COPD” [45]. In contrast, the Lancet Commission has indicated that these individuals have “COPD” despite the normal spirometric values [4]. In this paper, we have used the term “early COPD” to denote these individuals. Whatever the label, there is no evidence that the current inhaled therapeutics have any value in ameliorating symptoms in these patients. Although there is no universal consensus on the diagnostic criteria for early COPD, one proposal based on the Lancet Commission is shown in Figure 3.
A major challenge in developing novel therapies for early COPD is the lack of clear understanding of its pathogenesis. While airway inflammation is thought to play an important role in this process [46], the exact pathways relevant to this condition have not been elucidated. The prototypical anti-inflammatory drug, ICS, has been evaluated in smokers with or without COPD and shown to improve FEV1 slightly (by 50 to 100 mL) over 6 months and reduce patient symptoms such as exertional dyspnea or cough [47]. However, ICS does not modify the long term decline in FEV1 [48]. Thus, it is unknown whether ICS will be helpful in the management of early COPD.
While there are no specific therapies in development for early COPD, drugs targeting specific treatable traits of early COPD such as chronic cough are in therapeutic trials. For example, P2X purinoceptor 3 receptor antagonists are in phase 3 trials for treatment of idiopathic chronic cough [49]. Since cough is a very common symptom in smokers with preserved spirometry, these drugs may be effective in patients with early COPD. Other target drugs are being identified through the use of large scale genetics studies powered on various treatable traits. This genetics-based approach to target discovery holds great promise in identifying novel therapeutic targets for COPD and other chronic conditions [50].
Early COPD is likely very common among current and former smokers and is likely associated with significant symptom burden and health care service utilization. However, because by definition, these patients do not demonstrate significant airflow limitation on spirometry, other more sensitive technologies are required to make this diagnosis. This includes the use of imaging studies such as CT and hyperpolarized gas MRI, and PFTs such as impulse oscillometry, LCI, and body plethysmography and cardiopulmonary exercise tests (Table 2). Accurate diagnosis of early COPD is essential for disease modification as by the time patients demonstrate significant airflow limitation on spirometry, approximately 50% of small airways are lost and the remaining airways have become diseased. There is also a pressing need to develop therapeutics that can modify COPD at its earliest stages in order to eliminate this disease in the future.
Fig. 1.
Lung function trajectory of persons with and without chronic obstructive pulmonary disease (COPD). Lung function trajectory is characterized by lung growth and development until approximately 20 to 25 years of age (phase 1); after which lung function remains stable for approximately 10 years (phase 2) and then declines in an age-related way (phase 3). COPD can develop from perturbations in any of these phases. A combination of lung underdevelopment (a phase 1 abnormality) and accelerated decline in lung function (a phase 2 or 3 abnormality) results in the greatest risk for severe COPD and young COPD. The figure is based on data from Lange et al. [5]
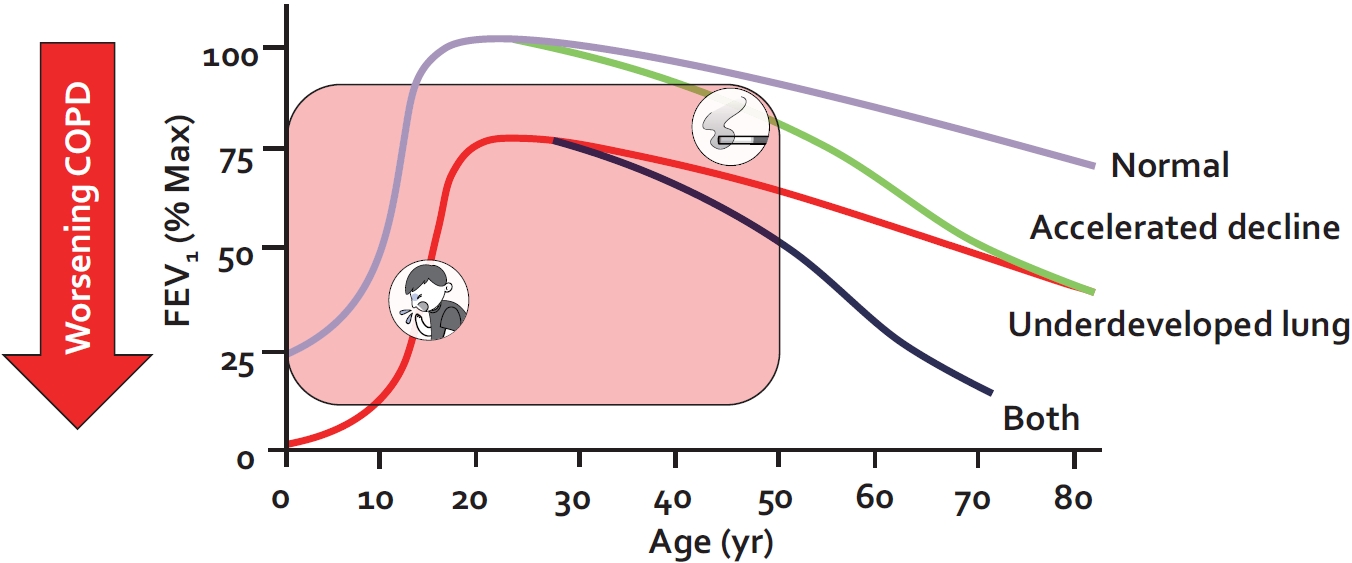
Fig. 2.
Rates of exacerbation and exercise capacity of smokers with preserved spirometry versus patients with mild to moderate chronic obstructive pulmonary disease (COPD). Exacerbation is defined as worsening of respiratory symptoms that leads to the prescription of antibiotics and/or prednisone; severe exacerbation is defined as worsening of respiratory symptoms leading to emergency visit and/or hospitalization. Exacerbations and severe exacerbations are expressed as the mean number of events per person per year. The 6-minute walk test is expressed as percent of predicted. Symptomatic smoker is defined by COPD Assessment Test (CAT) score of 10 or greater; Nonsymptomatic smoker is defined by CAT score of less than 10. The data are from Woodruff et al. [41]
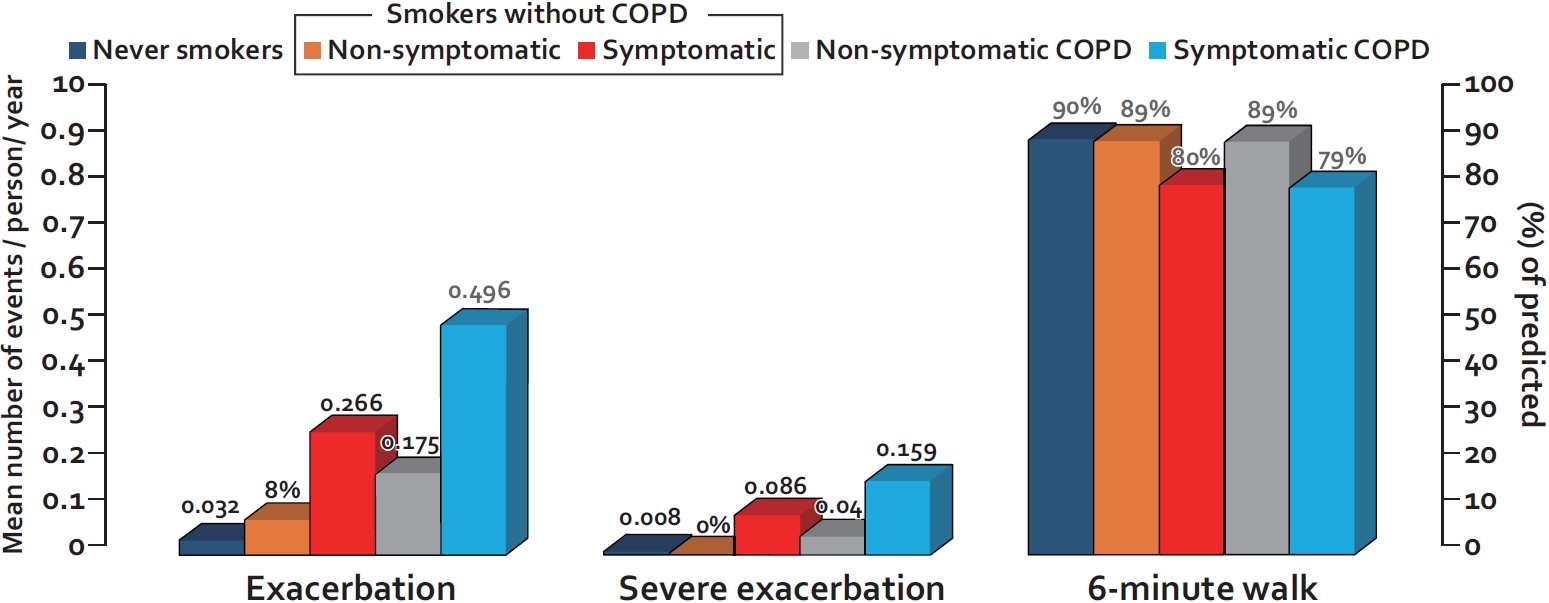
Fig. 3.
Diagnostic approach to early chronic obstructive pulmonary disease (COPD). Persons with a significant risk factor for COPD (e.g., smokers, childhood asthma, air pollution exposed, etc.) should first be tested with spirometry. If spirometry is preserved, then they should be classified into “symptomatic” or “non-symptomatic” group based on a COPD Assessment Test (CAT) score threshold of 10. For symptomatic persons, additional testing should be considered including body plethysmography, computed tomography (CT) or magnetic resonance imaging, impulse oscillometry (IOS), and/ or lung clearance index (LCI). A significant abnormality in any of these tests likely denotes “Early” COPD. The approach was adopted from Stolz et al.4 PFT: pulmonary function test; CPET: cardiopulmonary exercise testing.
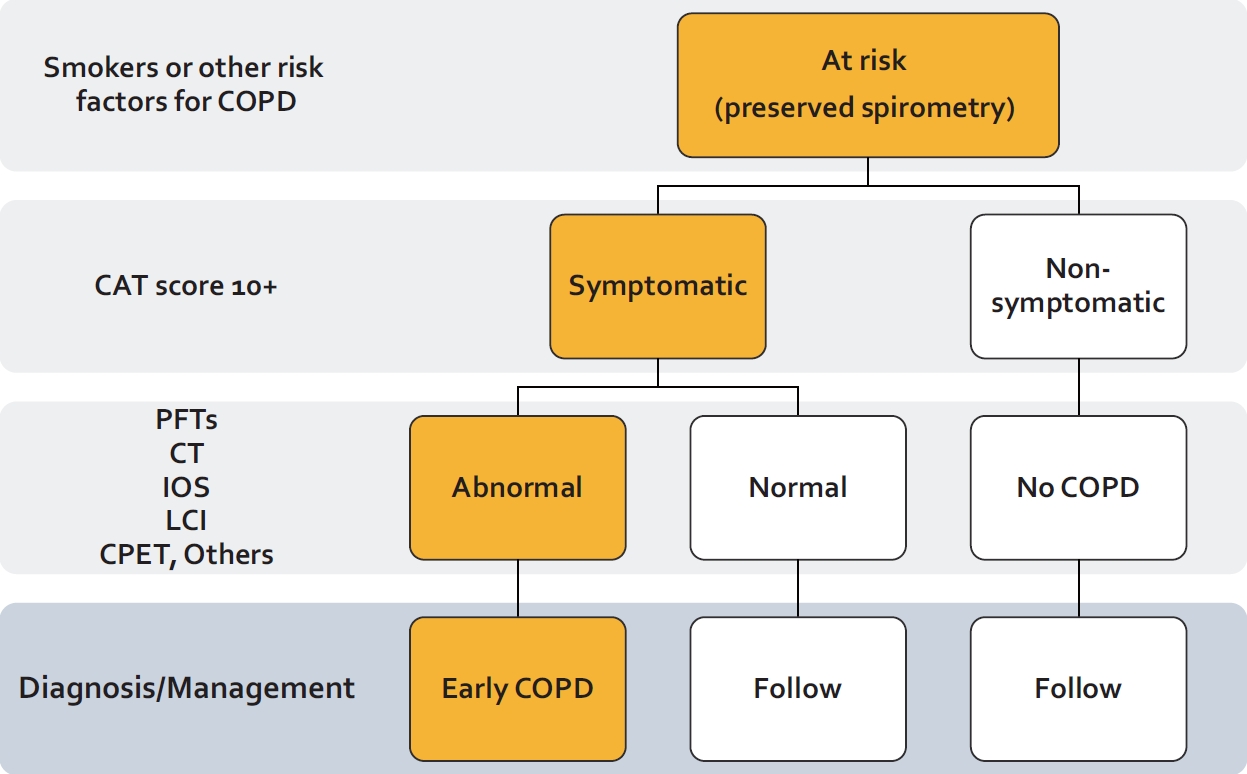
Table 1.
Definitions
Table 2.
Methods to detect small and large airways abnormalities in early COPD
COPD: chronic obstructive pulmonary disease; FEF25%-75%: mean forced expiratory flow during the middle half of the FVC; FEF50%: mean forced expiratory flow at 50% FVC; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; RV/TLC: residual volume to total lung capacity; DLCO: diffusing capacity of lung for carbon monoxide; AX: reactance area; R5: resistance at 5 Hz; R20: resistance at 20 Hz; Sacin: acinar ventilation heterogeneity; Scond: conductive airways ventilation heterogeneity; LCI: lung clearance index; CV: closing volume; CC: closing capacity; CT: computed tomography; PRM: parametric response mapping; MRI: magnetic resonance imaging; VDP: ventilation defect percent; ADP: apparent diffusion percent.
REFERENCES
1. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022;10:447-58.



2. Institute for Health Metrics and Evaluation. GBD compare: Viz Hub [Internet]. Seattle: IHME; 2019 [cited 2023 Feb 27]. Available from: https://vizhub.healthdata.org/gbd-compare/.
3. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223-49.


4. Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022;400:921-72.


5. Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015;373:111-22.


6. Agusti A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J 2023;3:2300239.
7. Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J 2018;52:1801448.


8. Martinez FJ, Agusti A, Celli BR, Han MK, Allinson JP, Bhatt SP, et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med 2022;205:275-87.


9. Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med 2006;355:21-30.


11. Tan WC, Bourbeau J, Aaron SD, Hogg JC, Maltais F, Hernandez P, et al. The effects of marijuana smoking on lung function in older people. Eur Respir J 2019;54:1900826.


12. Tan WC, Lo C, Jong A, Xing L, Fitzgerald MJ, Vollmer WM, et al. Marijuana and chronic obstructive lung disease: a population-based study. CMAJ 2009;180:814-20.



13. Ling SH, McDonough JE, Gosselink JV, Elliott WM, Hayashi S, Hogg JC, et al. Patterns of retention of particulate matter in lung tissues of patients with COPD: potential role in disease progression. Chest 2011;140:1540-9.


14. Ural BB, Caron DP, Dogra P, Wells SB, Szabo PA, Granot T, et al. Inhaled particulate accumulation with age impairs immune function and architecture in human lung lymph nodes. Nat Med 2022;28:2622-32.




15. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA 1994;272:1497-505.


16. Koo HK, Vasilescu DM, Booth S, Hsieh A, Katsamenis OL, Fishbane N, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med 2018;6:591-602.


17. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709-21.


18. Kwon DS, Choi YJ, Kim TH, Byun MK, Cho JH, Kim HJ, et al. FEF25-75% values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2020;15:2913-21.


19. Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J 2014;43:1051-8.


20. King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellaca RL, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020;55:1900753.


21. Kaminsky DA, Simpson SJ, Berger KI, Calverley P, de Melo PL, Dandurand R, et al. Clinical significance and applications of oscillometry. Eur Respir Rev 2022;31:210208.



22. Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 2011;105:1069-78.


23. Jetmalani K, Thamrin C, Farah CS, Bertolin A, Chapman DG, Berend N, et al. Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology 2018;23:512-8.



24. Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med 2019;7:402-16.


25. Kraft M, Richardson M, Hallmark B, Billheimer D, Van den Berge M, Fabbri LM, et al. The role of small airway dysfunction in asthma control and exacerbations: a longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respir Med 2022;10:661-8.


26. Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41:507-22.


27. Olofson J, Bake B, Bergman B, Vanfleteren LE, Svardsudd K. Prediction of COPD by the single-breath nitrogen test and various respiratory symptoms. ERJ Open Res 2021;7:00383-2021.



28. Olofsson J, Bake B, Svardsudd K, Skoogh BE. The single breath N2-test predicts the rate of decline in FEV1: the study of men born in 1913 and 1923. Eur J Respir Dis 1986;69:46-56.

29. Olofson J, Bake B, Bergman B, Svardsudd K. The single breath nitrogen test and mortality: a 38 years follow up. Respir Med 2016;112:75-80.


30. Pistelli F, Sherrill DL, Di Pede F, Baldacci S, Simoni M, Maio S, et al. Single breath nitrogen test as predictor of lung function decline and COPD over an 8-year follow-up. Pulmonology 2022 Oct 7 [Epub]. https://doi.org/10.1016/j.pulmoe.2022.09.001.

31. Buist AS, Vollmer WM, Johnson LR, McCamant LE. Does the single-breath N2 test identify the smoker who will develop chronic airflow limitation? Am Rev Respir Dis 1988;137:293-301.


32. Elbehairy AF, Guenette JA, Faisal A, Ciavaglia CE, Webb KA, Jensen D, et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur Respir J 2016;48:694-705.


33. Washko GR, Parraga G. COPD biomarkers and phenotypes: opportunities for better outcomes with precision imaging. Eur Respir J 2018;52:1801570.


34. Yuan R, Hogg JC, Pare PD, Sin DD, Wong JC, Nakano Y, et al. Prediction of the rate of decline in FEV(1) in smokers using quantitative computed tomography. Thorax 2009;64:944-9.


35. Arjomandi M, Zeng S, KirBarjaktarevicby I, Barr RG, Bleecker ER, Bowler RP, et al. Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur Respir J 2019;541802214.

36. Kirby M, Tanabe N, Vasilescu DM, Cooper JD, Mc-Donough JE, Verleden SE, et al. Computed tomography total airway count is associated with the number of micro-computed tomography terminal bronchioles. Am J Respir Crit Care Med 2020;201:613-5.


37. Tang LY, Coxson HO, Lam S, Leipsic J, Tam RC, Sin DD. Towards large-scale case-finding: training and validation of residual networks for detection of chronic obstructive pulmonary disease using low-dose CT. Lancet Digit Health 2020;2:e259-67.


38. Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18:1711-5.




39. Ruppert K, Qing K, Patrie JT, Altes TA, Mugler JP 3rd. Using hyperpolarized Xenon-129 MRI to quantify early-stage lung disease in smokers. Acad Radiol 2019;26:355-66.


40. Baron RJ, Hamedani H, Kadlecek SJ, Duncan IF, Xin Y, Siddiqui S, et al. A model for predicting future FEV1 decline in smokers using hyperpolarized 3He magnetic resonance imaging. Acad Radiol 2019;26:383-94.


41. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016;374:1811-21.



43. Han MK, Ye W, Wang D, White E, Arjomandi M, Barjaktarevic IZ, et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med 2022;387:1173-84.



44. Sin DD. RETHINCking COPD: bronchodilators for symptomatic tobacco-exposed persons with preserved lung function? N Engl J Med 2022;387:1230-1.


45. Celli B, Fabbri L, Criner G, Martinez FJ, Mannino D, Vogelmeier C, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med 2022;206:1317-25.



46. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53.


47. Lung Health Study Research Group, Wise R, Connett J, Weinmann G, Scanlon P, Skeans M. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-9.


48. Soriano JB, Sin DD, Zhang X, Camp PG, Anderson JA, Anthonisen NR, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest 2007;131:682-9.


49. McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022;399:909-23.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




