 |
 |
| Tuberc Respir Dis > Volume 86(2); 2023 > Article |
|
Abstract
Notes
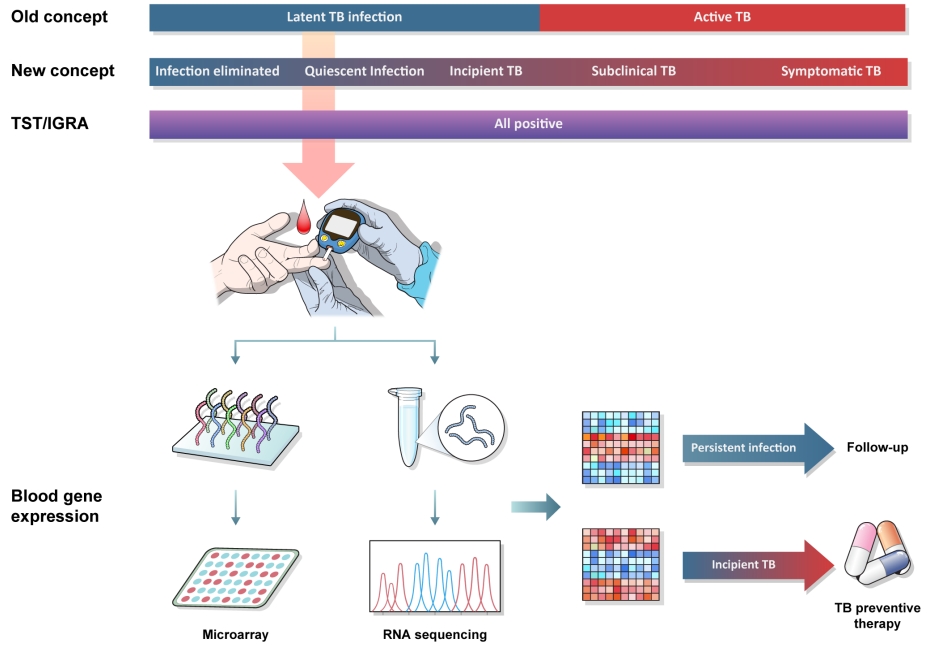
Fig.┬Ā1.
Table┬Ā1.
| Study | Target gene signatures | Study population | Performance, AUC (95% CI)/time interval prior to diagnosis | Achievement of performance criteria based on the WHO TPP | Study design |
|---|---|---|---|---|---|
| Zak et al. [23] (2016) | Zak16 | South African adolescent latent TB cohort (ACS), South African and Gambian household contacts (GC6-74) | 0.72 (0.64-0.80) to 0.78 (0.76-0.80)/12 mo | None | Prospective cohort study |
| Suliman et al. [24] (2018) | RISK4 | GC6-74, ACS | 0.66 (0.55-0.78)/12 mo | None | Case-control study |
| 0.69 (0.51-0.86)/12-24 mo | |||||
| Roe et al. [26] (2020) | Roe3 | UK TB contacts | 0.96 (0.92-1.0)/3 mo 0.70 (0.64-0.76) to | NA | Preexisting dataset plus new cohort data |
| Gupta et al. [25] (2020) | BATF2, Roe3, Sweeney3, Gliddon3, Suliman2, Suliman4, Zak16, Kaforou25 | ACS, GC6-74, UK TB contacts | 0.77 (0.71-0.82)/24 mo | None (except Sweeney3 [3 mominimum]) | Pooled dataset from published studies |
| Scriba et al. [27] (2021) | RISK11 | South African community setting | 0.63 (0.47-0.80)/15 mo | None (except 6 mooptimum & 12 mominimum) | Prospective RCT |
| Mendelsohn et al. [28] (2021) | RISK11 | South African community setting (HIV) | 0.80 (0.71-0.87)/15 mo | None | Prospective cohort study |
AUC: area under the receiver operating characteristic curve; CI: confidence interval; WHO: World Health Organization; TPP: target product profile; TB: tuberculosis; ACS: adolescent cohort study; GC6-74: Grand Challenges 6-74 study; NA: not applicable; RCT: randomized controlled trial; HIV: human immunodeficiency virus.
REFERENCES
-
METRICS

- ORCID iDs
-
Chang Ho Kim

https://orcid.org/0000-0002-1550-5752Gahye Choi

https://orcid.org/0000-0002-7810-1835Jaehee Lee

https://orcid.org/0000-0001-8111-7320 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



