 |
 |
| Tuberc Respir Dis > Volume 73(6); 2012 > Article |
|
Abstract
Primary effusion lymphoma (PEL) is a rare type of lymphoma that arises in the body cavity without detectable masses. It is associated with human herpes virus-8 (HHV-8), Epstein-Barr virus (EBV), and human immunodeficiency virus (HIV). Recently, PEL unrelated to viral infection has been reported and it has been termed HHV-8 unrelated primary effusion lymphoma-like lymphoma (HHV-8 unrelated PEL-like lymphoma). Here, we report a case of HHV-8 unrelated PEL-like lymphoma in an 80-year-old woman. Chest X-ray and computed tomography revealed left-sided pleural effusion. Pleural effusion analysis and mediastinoscopic biopsy showed atypical cells that had originated from the B cells. The cells were positive for CD20 and bcl-2, but negative for CD3, CD5, CD21, CD30, CD138, epithelial membrane antigen, and HHV-8. Serological tests for HIV and EBV were negative. Considering the patient's age, further treatments were not performed. She has shown good prognosis without chemotherapy for more than 18 months.
Primary effusion lymphoma (PEL) is a rare subtype of non-Hodgkin's lymphoma that involves body cavities, causing serous effusions without detectable masses or lymphadenopathy. It is associated with human herpes virus-8 (HHV-8), Epstein-Barr virus (EBV), and human immunodeficiency virus (HIV)1,2. The World Health Organization (WHO) classified it as a clinical subtype of B cell lymphoma3. However, PEL unrelated to the above viruses has been reported4. It has been termed HHV-8 unrelated primary effusion lymphoma-like lymphoma (HHV-8 unrelated PEL-like lymphoma). Until now, HHV-8 unrelated PEL-like lymphoma cases were rarely reported, and their prognosis differ from PEL associated with viruses described above. We experienced a case of HHV-8 unrelated PEL-like lymphoma in an 80-year-old woman.
An 80-year-old Korean woman was admitted to our hospital on April 13, 2009, complaining of experiencing exertional dyspnea, cough, and sputum production in the past two weeks. She had been treated for hypertension three years ago, and family history was non specific.
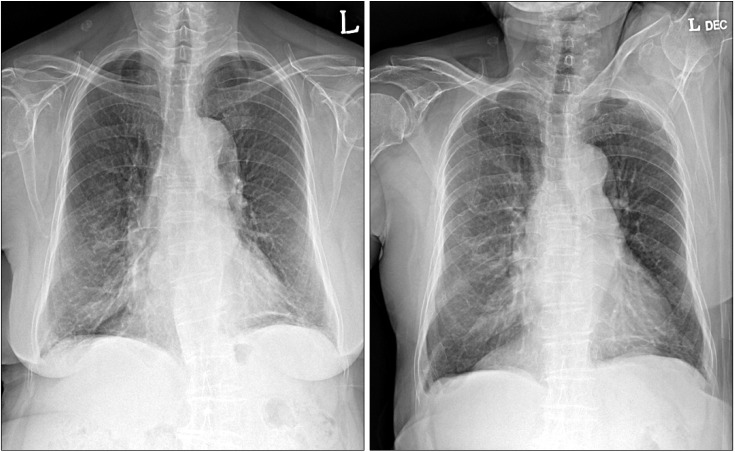
Physical examination revealed that breath sounds on the left lower hemithorax were decreased. The liver, spleen, and superficial lymph nodes were not palpable. Hemogram revealed a leukocyte of 6,000/mm3, a hemoglobin of 12.2 g/dL and a platelet count of 224,000/mm3. The blood chemistry profile showed the following: total protein level 7.6 g/dL, albumin 2.9 g/dL, total bilirubin 0.41 mg/dL, aspartate aminotransferase 21 IU/L, alanine aminotransferase 13 IU/L, alkaline phosphatase 309 IU/L, lactate dehydrogenase (LDH) 377 IU/L, blood urea nitrogen 12.1 mg/dL, and creatinine 0.7 mg/dL. Chest X-ray and computed tomography of chest revealed left pleural effusion (Figure 1). The analysis of left pleural fluid revealed the following: pH 8.0, protein 5.8 g/dL, and LDH 12,580 IU/dL. Cell count of the pleural fluid showed a leukocyte of 3,400/mm3 (77% lymphocytes). Pleural fluid adenosine deaminase (ADA) was 131 IU/L. Cytological evaluation of the pleural fluid indicated that no malignant cells were present. She was diagnosed with tuberculous pleural effusion, and first-line anti-tuberculous drugs were administered. Three months after taking anti-tuberculous drugs, the volume of pleural effusion increased again. Thoracentesis and pleural biopsy were performed. It showed lymphocyte-dominant exudate. Pleural fluid ADA was 88 IU/L. Cytological evaluation of the pleural fluid indicated many lymphoid cells were seen and no malignant cells were present. Pleural biopsy also showed no malignant cells. She was diagnosed for paradoxical response after the treatment of tuberculosis. Therefore, anti-tuberculous drugs were administered continuously.
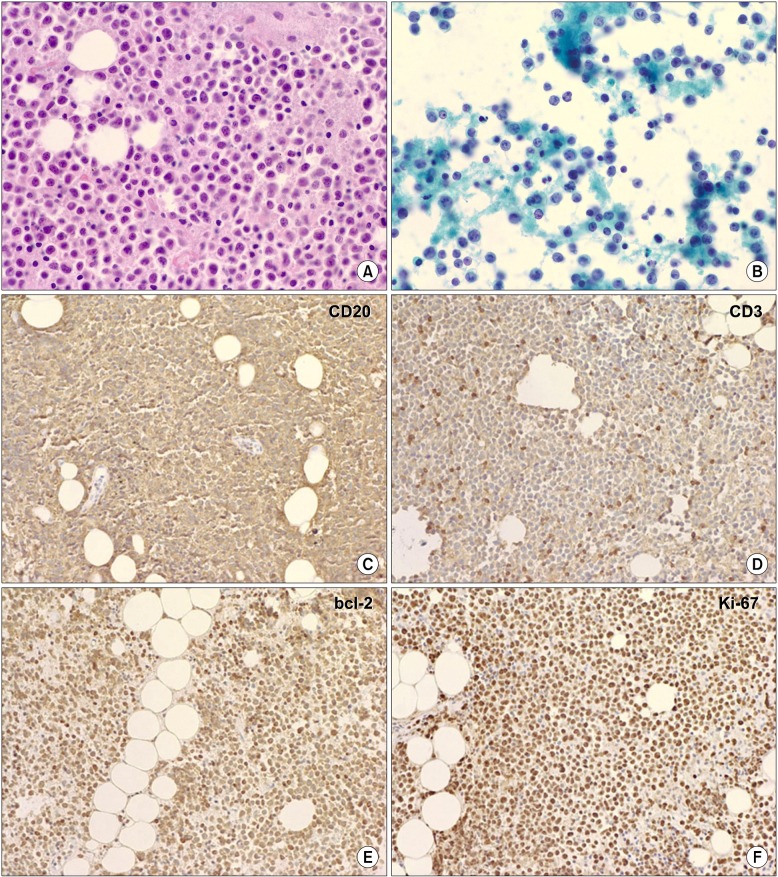
Six months after the initial administration of anti-tuberculous drugs, the volume of pleural effusion increased again. Thorough analysis of the pleural fluid followed. The analysis of left pleural fluid revealed the following: pH 7.5, protein 3.1 g/dL, and LDH 7,460 IU/dL. Cell count of the pleural fluid showed a leukocyte of 12,750/mm3. Pleural fluid ADA was 52 IU/L. Cytological evaluation of the pleural fluid showed many atypical lymphoid cells having medium to large nuclei and nucleoli. This suggested B lymphoid cell malignancy is possible. So we performed positron emission tomography-computed tomography scan. It showed strong fluorodeoxyglucose uptake in the mediastinal pleura (Figure 2). Histological tests were performed on the mediastinal pleura and adjacent tissues by mediastinoscopy. The biopsy finding of the pleura revealed the infiltration of atypical cells to fibrotic tissues (Figure 3A, B). These cells were positive for CD20 and bcl-2, but negative for CD3, CD5, CD21, CD30, CD138, and epithelial membrane antigen (EMA). The cells also showed high Ki-67 (60~70%) (Figure 3C~F). The tests for HHV-8 in the tissue were negative.
Finally, she was diagnosed as HHV-8 unrelated primary effusion lymphoma-like lymphoma. In serological tests, HIV, hepatitis C virus (HCV), and EBV-polymerase chain reaction (EBV-PCR) were negative. Considering her old age, further treatments were not performed. She has shown good prognosis despite receiving no chemotherapy for more than 18 months (Figure 4).
PEL is a lymphoma originating from B cells, and for 0.5~2% of the cases of non-Hodgkin's lymphomas. It can occur in any body cavities without detectable masses or lymphadenopathy. It has been reported primarily in patients infected with HHV-8, EBV, or HIV1,2. According to the WHO, the term PEL describes the cases associated with HHV-8. The cases unrelated to the virus were termed HHV-8 unrelated PEL-like lymphoma. The diagnosis of PEL is usually made on a cytological preparation of the involved effusion fluid, but biopsies of body cavity-lining tissue might also show small numbers of neoplastic cells. Detecting evidence of viral infection by HHV-8 in the neoplastic cells is also essential for the diagnosis of PEL3. When PEL is not related to the virus, we diagnosed that case as a HHV-8 unrelated PEL-like lymphoma. In our case, cytological evaluation of the pleural fluid showed atypical lymphoid cells. These cells were positive for CD20 and bcl-2, but negative for CD3, CD5, CD21, CD30, CD138, and EMA. The tests for HHV-8 in the tissue and serological test for HIV, HCV, and EBV-PCR were negative. Presently, 31 cases of HHV-8 unrelated PEL have been reported worldwide4,5.
HHV-8 has been reported to play an important role in the etiology of PEL by causing the rearrangement and deletion of c-MYC gene associated with B cells1,5. In addition, the association with EBV has been occasionally reported1,2. But the definite pathogenesis of HHV-8 unrelated PEL-like lymphoma is still unknown. It has been reported that HCV, iatrogenic immunodeficiency, alcoholism, liver cirrhosis, cancer, and old age can be possible reasons for HHV-8 unrelated PEL-like lymphoma5-11.
In Korea, where the incidence of tuberculosis is high, HHV-8 unrelated PEL like lymphoma may be misdiagnosed as tuberculous pleurisy as in our case. In the initial effusion tapping of our case, lymphocyte was 77% and ADA was 131 IU/L. It has been reported that in most cases of tuberculous lymphocytic effusion, the level of ADA is higher than 40 IU/L12. Especially in Korea, where the incidence of tuberculous diseases is high, patients with high ADA and lymphocyte dominant pleural effusion are diagnosed with tuberculous diseases. Nevertheless, if conditions do not improved despite of the administration of anti-tuberculous drugs, aggressive tests should be performed due to the possibility of other diseases. In our case, invasive tests were difficult for the elderly patient who was 80 years old. However, we questioned the diagnosis of tuberculosis and performed the invasive tests, so that malignant lymphoma could be diagnosed. PEL progresses rapidly despite of treatments, and the median survival period is less than 3~6 months, making prognosis very poor4. However, as in our case or other HHV-8, EBV, or HIV unrelated PEL-like lymphoma cases, survival period of 40 months without special treatments has been reported5. The reason why HHV-8 unrelated PEL-like lymphoma has better prognosis than PEL has not yet been revealed. Two cases of HHV-8 negative PEL with spontaneous regression of the malignant cells without any treatment after aspirations of the serous effusion have been reported from Japan10,11. Terasaki et al.13 suggested that sufficient drainage of the serous effusion may induce cytogenetic complete remission in patients with HHV-8 unrelated PEL-like lymphoma. In our case, we assume that the reason why our patient has good prognosis is that the patient has no viral disease and any other severe comorbid illness. But it is not enough to explain the good prognosis of HHV-8 unrelated PEL-like lymphoma. Hence, further studies about the pathogenesis of HHV-8 unrelated PEL-like lymphoma should be conducted continuously along with its treatment.
References
1. Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 1996;88:645-656. PMID: 8695812.



2. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186-1191. PMID: 7700311.


3. Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist 2007;12:569-576. PMID: 17522245.


4. Banks PM, Warnke RA. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, Primary effusion lymphoma. editors. World Health Organization classification of tumors: pathology and genetics of tumors of haematopoietic and lymphoid tissues. 2001. Lyon: IARC Press; p. 179-180.
5. Adiguzel C, Bozkurt SU, Kaygusuz I, Uzay A, Tecimer T, Bayik M. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. APMIS 2009;117:222-229. PMID: 19245595.


6. Tanaka S, Katano H, Tsukamoto K, Jin M, Oikawa S, Nishihara H, et al. HHV8-negative primary effusion lymphoma of the peritoneal cavity presenting with a distinct immunohistochemical phenotype. Pathol Int 2001;51:293-300. PMID: 11350613.


7. Carbone A, Gloghini A. PEL and HHV8-unrelated effusion lymphomas: classification and diagnosis. Cancer 2008;114:225-227. PMID: 18473348.


8. Takao T, Kobayashi Y, Kuroda J, Omoto A, Nishimura T, Kamitsuji Y, et al. Rituximab is effective for human herpesvirus-8-negative primary effusion lymphoma with CD20 phenotype associated hepatitis C virus-related liver cirrhosis. Am J Hematol 2004;77:419-420. PMID: 15551361.


9. Matsumoto Y, Nomura K, Ueda K, Satoh K, Yasuda N, Taki T, et al. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leuk Lymphoma 2005;46:415-419. PMID: 15621832.


10. Ichinohasama R, Miura I, Kobayashi N, Saitoh Y, DeCoteau JF, Saiki Y, et al. Herpes virus type 8-negative primary effusion lymphoma associated with PAX-5 gene rearrangement and hepatitis C virus: a case report and review of the literature. Am J Surg Pathol 1998;22:1528-1537. PMID: 9850179.


11. Inoue S, Miyamoto T, Yoshino T, Yamadori I, Hagari Y, Yamamoto O. Primary effusion lymphoma with skin involvement. J Clin Pathol 2006;59:1221-1222. PMID: 17071811.



12. Lee YC, Rogers JT, Rodriguez RM, Miller KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest 2001;120:356-361. PMID: 11502629.


13. Terasaki Y, Okumura H, Saito K, Sato Y, Yoshino T, Ichinohasama R, et al. HHV-8/KSHV-negative and CD20-positive primary effusion lymphoma successfully treated by pleural drainage followed by chemotherapy containing rituximab. Intern Med 2008;47:2175-2178. PMID: 19075546.


Figure 1
Chest X-ray (A, B) and computed tomography (C) at the time of admission showed left pleural effusion without evidence of pleural masses.

Figure 2
Positron emission tomography-computed tomography showed multiple focal strong fluorodeoxyglucose uptakes in mediastinal pleura adjacent to right upper lobe and right lower lobe, pretracheal node bearing areas.
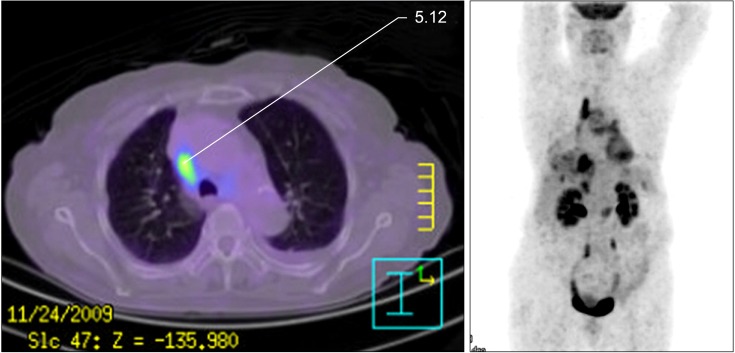
Figure 3
(A) Thoracoscopic biopsy showed diffuse infiltration of large atypical lymphoid cells (H&E stain, ×200). (B) Pleural cytology showed many atypical lymphoid cells having medium to large nuclei and nucleoli. Frequent mitoses were found (Papanicolaou stain, ×200). (C~F) The tumor cells were positive for CD20. Immunohistochemistry showed CD20 (C), CD3 (D, negative), bcl-2 (E, positive), and Ki-67 (F, high-proliferating index) (×100).


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




