 |
 |
| Tuberc Respir Dis > Volume 84(3); 2021 > Article |
|
Abstract
Background
Methods
Results
Supplementary Material
Notes
Figure 1
Figure 2
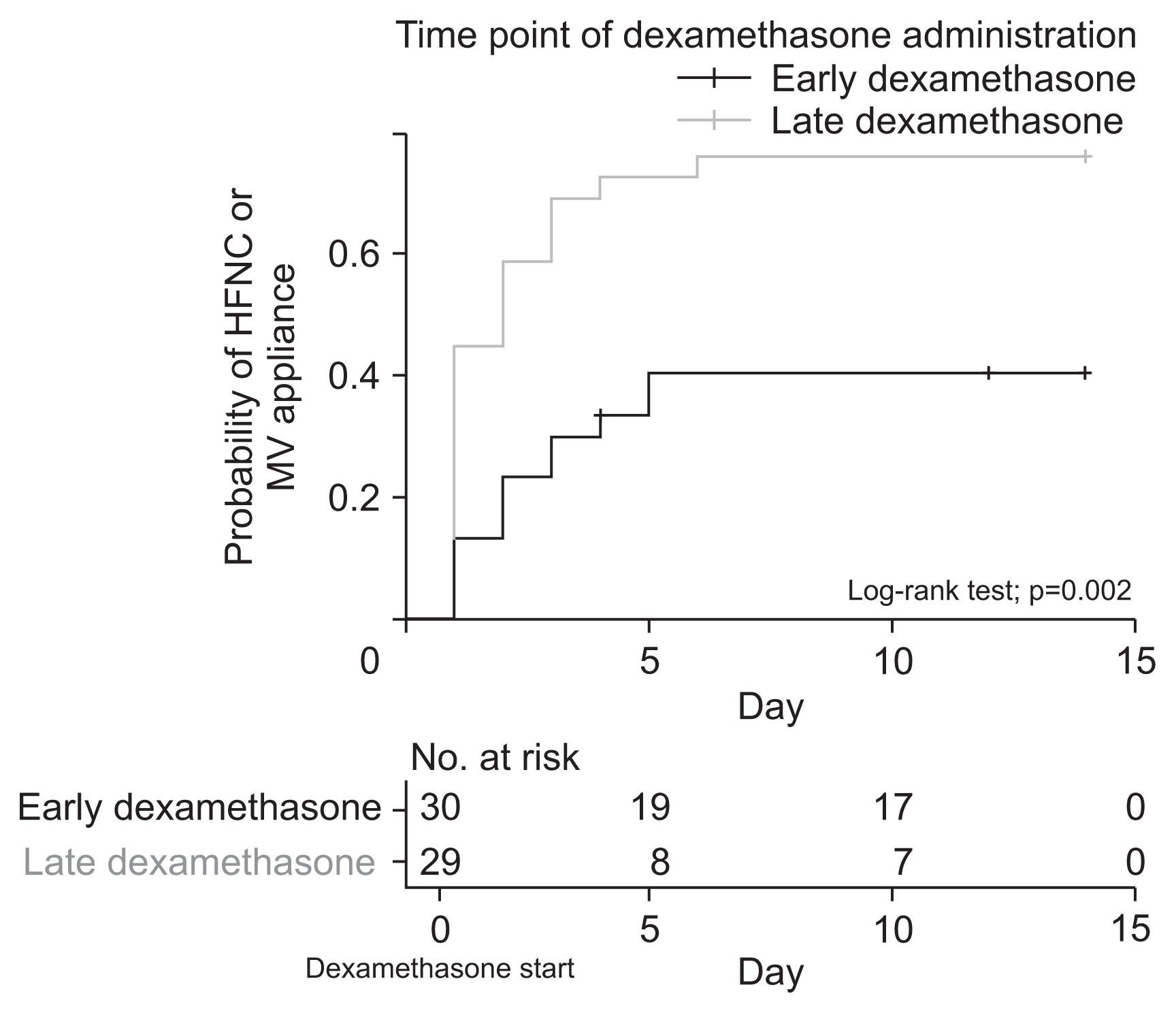
Table 1
Table 2
| Variable | Late dexamethasone group (n=29) | Early dexamethasone group (n=30) | p-value |
|---|---|---|---|
| Clinical features | |||
| Ct-value of RT-PCR at first diagnosis | |||
| E gene | 22.07±6.32 | 21.65±4.92 | 0.786 |
| RdRP gene | 21.80±6.49 | 20.36±5.60 | 0.382 |
| Clinical severity | |||
| SOFA score at hypoxemia development | 3.5±1.8 | 4.6±2.2 | 0.044 |
| SAPS II at hypoxemia development | 21.1±6.2 | 26.7±9.7 | 0.010 |
| SpO2 at hypoxemia development | 88.9±4.9 | 87.0±3.1 | 0.081 |
| PaO2/FiO2 ratio at hypoxemia development | 296±117 | 202±63 | <0.001 |
| Laboratory test at hypoxemia development | |||
| White blood cell counts, ×103/μL | 5,963±3,705 | 6,490±2,377 | 0.517 |
| The number of lymphocytes, ×103/μL | 946±376 | 1,018±499 | 0.533 |
| C-reactive protein, mg/dL | 7.50±6.34 | 9.59±6.50 | 0.217 |
| Procalcitonin, ng/mL | 0.05 (0.04-0.08) | 0.09 (0.05-0.21) | 0.181 |
| Lactate dehydrogenase, IU/L | 293 (220-376) | 367 (303-450) | 0.111 |
| Troponin-I, ng/mL | 9.1 (4.2-13.8) | 10.2 (5.2-19.2) | 0.124 |
| D-dimer, mg/L | 0.8 (0.5-2.1) | 0.8 (0.6-1.6) | 0.640 |
| Treatment | |||
| Remdesivir | 13 (44.8) | 12 (40.0) | 0.911 |
| Antibiotics | 29 (100) | 30 (100) | NA |
| Total accumulative dose of dexamethasone mg | 62.62±33.34 | 64.17±32.48 | 0.857 |
| Total duration of dexamethasone mg | 10.66±5.58 | 10.57±4.48 | 0.947 |
| Time interval from symptom onset to dexamethasone administration, day | 7.76±3.59 | 7.03±3.50 | 0.435 |
| Time interval from diagnosis to dexamethasone administration, day | 4.14±3.26 | 4.70±3.67 | 0.537 |
| Time interval from hypoxemia to dexamethasone administration, day | 2 (1-2) | 0 (0-0) | <0.001 |
| Clinical outcomes | |||
| High-flow nasal cannula or mechanical ventilation | 22 (75.9) | 12 (40.0) | 0.012 |
| Extracorporeal membrane oxygenation | 2 (6.9) | 0 (0) | 0.457 |
| Total duration of oxygen supplementation*,†, day | 21.61±16.42 | 10.45±9.39 | 0.003 |
| Length of stay in hospital†, day | 27.21±13.28 | 19.76±8.05 | 0.013 |
| In-hospital death | 1 (3.4) | 1 (3.3) | >0.990 |
* Oxygen supplementation includes oxygen inhalation through nasal prong, facial mask, high-flow nasal cannula, mechanical ventilator, and extracorporeal membrane oxygenation.
† Data regarding total duration of oxygen supplementation and length of stay in hospital were missing for 1 patient in early dexamethasone group and 1 patient in late dexamethasone group.
COVID-19: coronavirus disease 2019; RT-PCR: reverse transcription polymerase chain reaction; SOFA: Sequential Organ Failure Assessment; SAPS: Simplified Acute Physiology Score; SpO2: pulse oximeter oxygen saturation; PaO2/FiO2 ratio: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen.
Table 3
In model 1, early dexamethasone (initiation time of dexamethasone treatment within 24 hours) was used as a categorical variable for multivariable analysis. In model 2, initiation time of dexamethasone treatment was used as a continuous variable for multivariable analysis. multivariable dox regression analysis was adjusted by covariates including SOFA score and SAPS II.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hyun Woo Lee

https://orcid.org/0000-0003-4379-0260Eun Young Heo

https://orcid.org/0000-0003-3803-4903 - Related articles
-
The Effect of External PEEP on Work of Breathing in Patients with Auto-PEEP.1996 April;43(2)
The Effect of Pulmonary Rehabilitation in Patients with Chronic Lung Disease.1996 October;43(5)


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Supplement
Supplement Print
Print Download Citation
Download Citation



