Reliability of Portable Spirometry Performed in the Korea National Health and Nutrition Examination Survey Compared to Conventional Spirometry
Article information
Abstract
Background
The Korea National Health and Nutrition Examination Survey (KNHANES) is a well-designed survey to collect national data, which many researchers have used for their studies. In KNHANES, although portable spirometry was used, its reliability has not been verified.
Methods
We prospectively enrolled 58 participants from four Korean institutions. The participants were classified into normal pattern, obstructive pattern, and restrictive pattern groups according to their previous spirometry results. Lung function was estimated by conventional spirometry and portable spirometry, and the results were compared.
Results
The intraclass correlation coefficients of forced vital capacity (FVC) (coefficient, 9.993; 95% confidence interval [CI], 0.988–0.996), forced expiratory volume in 1 second (FEV1) (coefficient, 0.997; 95% CI, 0.995–0.998), FEV1/FVC ratio (coefficient, 0.995; 95% CI, 0.992–0.997), and forced expiratory flow at 25–75% (FEF25–75%; coefficient, 0.991; 95% CI, 0.984–0.994) were excellent (all p<0.001). In the subgroup analysis, the results of the three parameters were similar in all groups. In the overall and subgroup analyses, Pearson’s correlation of all the parameters was also excellent in the total (coefficient, 0.986–0.994; p<0.001) and subgroup analyses (coefficient, 0.915–0.995; p<0.001). In the paired t-test, FVC, FEV1/FVC, and FEF25–75% estimated by the two instruments were statistically different. However, FEV1 was not significantly different.
Conclusion
Lung function estimated by portable spirometry was well-correlated with that estimated by conventional spirometry. Although the values had minimal differences between them, we suggest that the spirometry results from the KNHANES are reliable.
Introduction
The Korea National Health and Nutrition Examination Survey (KNHANES) is a well-designed survey of national data with a complex, multi-stage probability sample extraction1. It includes vast amount of data on demographics, underlying diseases, nutritional status, laboratory data, and even lung function. Recently, coronavirus, air pollutants, and climate changes have made respiratory medicine a focus point2–4. The KNHANES data have been widely used in researches on various fields including respiratory medicine5–7. Several researchers have revealed that data on the prevalence and clinical characteristics of diseases are reliable under the premise that KNHANES represents all Koreans. Using KNHANES, Yoo et al.8 have reported that the prevalence of chronic obstructive pulmonary disease (COPD) estimated by spirometry was 13.4%, and among them, only 2.4% had been clinically diagnosed with COPD. Additionally, Chung et al.9 have shown that the prevalence of a restrictive spirometric pattern was 12.2%.
In the KNHANES, portable spirometry has been used to estimate lung function since July 2016. Portable spirometry is inexpensive, lightweight, and convenient to use, so that it has been used to establish the KNHANES data by conducting it in a moving bus. In the clinical setting, the non-movable conventional spirometry is widely used, so the reliability of the new portable equipment requires verification with clinical and scientific evidence10,11. However, whether the portable spirometry can be a substitute to conventional spirometry remains unclear. To accept study results from lung function data from the KNHANES, we need to confirm the reliability of portable spirometry. Thus, we aimed to verify whether data on lung function estimated by portable spirometry obtained through the KNHANES can be reliable.
Materials and Methods
1. Participants
We prospectively enrolled 58 participants from four Korean institutions. We included participants who admitted to respiratory clinics of university hospital from August 12, 2020 to November 11, 2020 and who are classified with following subgroups. Participants who cannot conduct spirometry were excluded. The participants were classified into normal pattern, obstructive pattern, and restrictive pattern groups according to their previous spirometry results. The normal pattern group was defined as participants without respiratory symptoms and underlying respiratory diseases. The obstructive pattern group was defined as those with underlying airway diseases including asthma or COPD and with an obstructive pattern in previous spirometry results (forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ratio <0.7)12. Lastly, the restrictive pattern group consisted of participants with underlying restrictive diseases including interstitial lung diseases and with a restrictive pattern in previous spirometry results (predicted FVC <80%)13.
2. Study design
The patients were admitted to the hospital and underwent a lung function test using two distinct equipment: conventional spirometry and portable spirometry. They rested at least 1 hour between the two tests. During the rest time, a short survey on demographics, symptoms, and medical history was conducted. The results estimated by the two equipment were compared.
3. Spirometry
The four participating institutions utilize the same non-portable conventional spirometry (Carefusion Vmax, SensorMedics, Milan, Italia) that measures lung function, which is widely used in Korea. These four institutions recruited almost same number of subjects evenly for each group. In two institutions, portable spirometry was conducted before conventional spirometry. In other two institutions, the order was reversed. Lung function tests were performed according to American Thoracic Society/European Respiratory Society recommendations14. Briefly, the following protocol was conducted. The patients were oriented with the test, and they were asked to slightly elevate their heads. They were made to wear a nose clip and a mouthpiece. They inhaled completely and rapidly with a short pause (<1 second), and then exhaled maximally until no more air can be expelled while maintaining an upright posture. The test was repeated as necessary (for a minimum of 3 maneuvers and a maximum of 8 maneuvers). The results met acceptability and reproducibility criteria.
4. Portable spirometry
The portable spirometer (Vyntus Spiro, Vyaire Medical GmbH, Hoechberg, Germany) was provided by the department of the KNHANES of the Korean Disease Control and Prevention Agency. Portable spirometry is constituted of light-weight pneumotach handle and long cable which can connect to private computer or notebook. From a single screen, patient data can be entered, flow sensor calibrated, test performed, quality assessment checked, and patient data trended via SentrySuite program. The detectable flow range is about zero to ±16 L/sec. Resolution is about 1 mL/sec and accuracy is about ±5% at 0.1 to 14 L/sec. Resistance is <0.05 kPa/L/sec at 10 L/sec.
5. Ethics
This study was approved by the ethics committee, Institutional Review Board (IRB) of four distinct institutions including Gangnam Severance Hospital (number: 3-2020-0264). Patient informed consent was obtained from all the participants.
6. Statistical analysis
Results are expressed as mean±standard error. Sample size (minimum number, 42) was calculated based on previous study (alpha level, 0.5 and power, 0.95)10. The values of FVC, FEV1, FEV1/FVC, and forced expiratory flow at 25–75% (FEF25–75%) were followed normal distribution, and it was justified by Shapiro-Wilk test or Kolmogorov-Smirnov test. The intraclass correlation coefficient (ICC) and Pearson’s correlation were used to assess the reproducibility and correlation between the results estimated by conventional and portable spirometry. ICC has been widely used to evaluate test-retest reliability. High ICC means high similarity between values form the same group. The significance of the different values estimated by two distinct types of spirometry was assessed using the paired t-test. Bland-Altman analysis was also used to compare two measurements. All statistical analyses were performed using the IBM SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). p-values <0.05 were considered statistically significant.
Results
1. Clinical characteristics of the patients
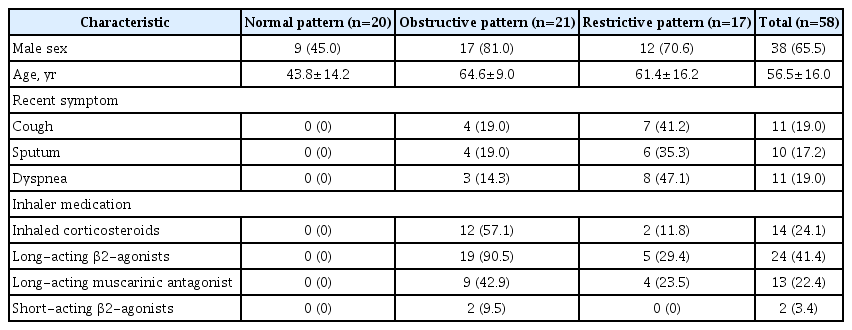
The normal pattern (n=20), obstructive pattern (n=21), and restrictive pattern (n=17) groups were evenly enrolled. Compared to the normal pattern group (male, 45.0%), the patients in the obstructive (81.0%) and restrictive (70.6%) pattern groups were predominantly male. Moreover, the mean age of the obstructive (64.6 years) and restrictive (61.4 years) pattern groups was higher than that of the normal pattern group (43.8 years). The normal pattern group had no respiratory symptoms and no history of inhaler use, in contrast to some patients in the other groups (Table 1).
2. Results of ICC
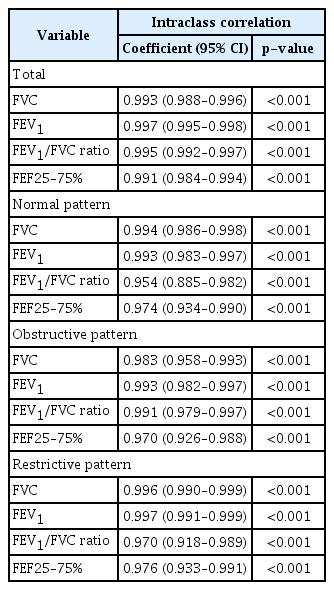
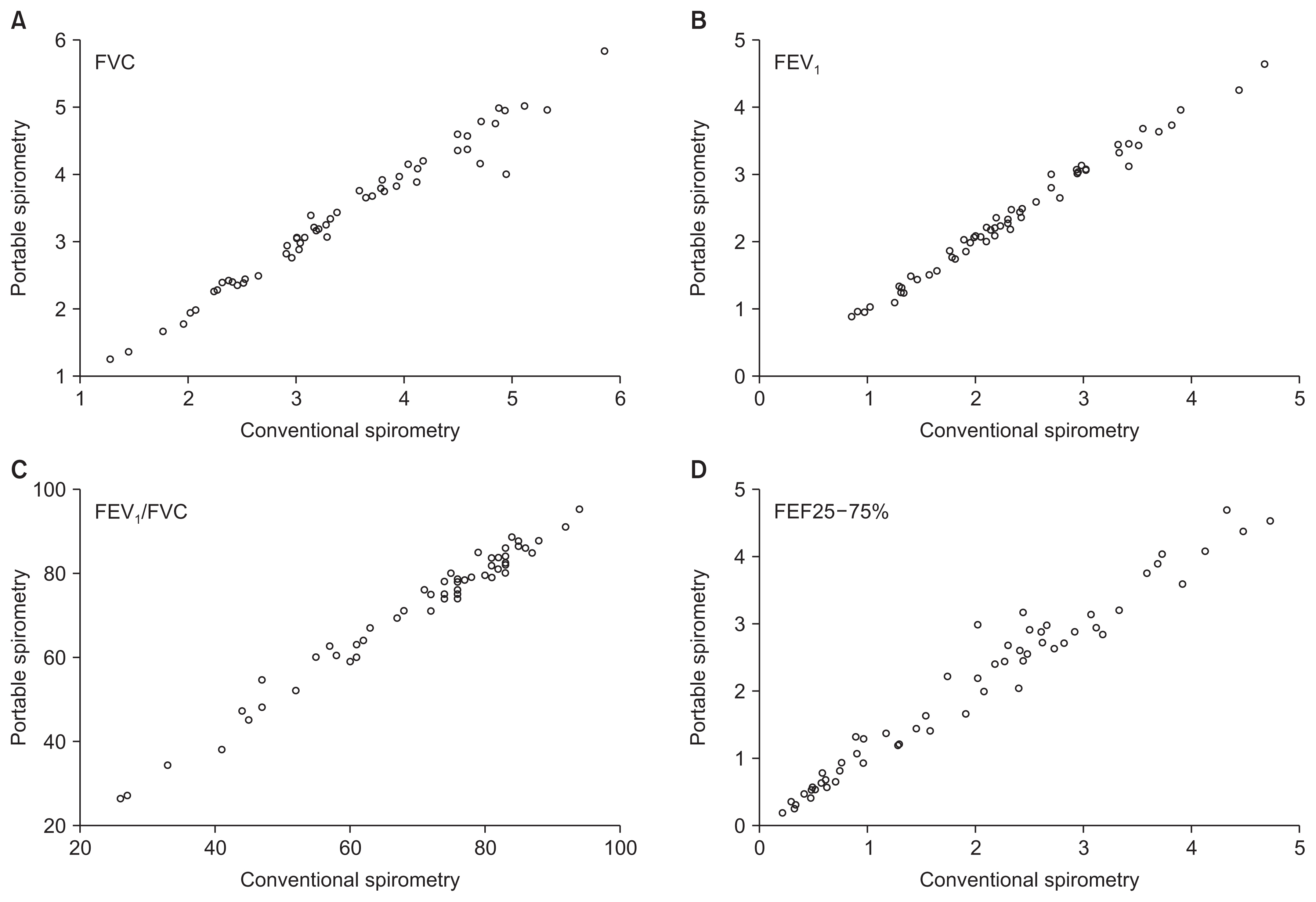
Altogether, the ICC of FVC (coefficient, 9.993; 95% confidence interval [CI], 0.988–0.996), FEV1 (coefficient, 0.997; 95% CI, 0.995–0.998), and FEV1/FVC ratio (coefficient, 0.995; 95% CI, 0.992–0.997) was excellent (all p<0.001). Similarly, in the subgroup analysis, the ICC of FVC, FEV1, FEV1/FVC, and FEF25–75% was also excellent (coefficient, 9.954–9.997; all p<0.001) (Table 2). The scattered plot shows the excellent correlation of all parameters (Figure 1).
3. Results of the Pearson’s correlation
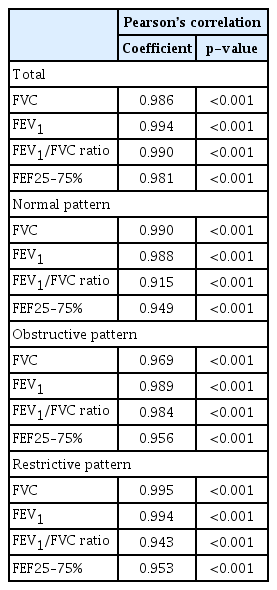
Altogether, the Pearson’s correlation of FVC (coefficient, 9.986), FEV1 (coefficient, 0.994), FEV1/FVC (coefficient, 0.990), and FEF25–75% (coefficient, 0.981) were excellent (all p<0.001). In the subgroup analysis, Pearson’s correlation was also excellent (coefficient, 9.915–9.995; all p<0.001) (Table 3).
4. Comparison of values measured by conventional and portable spirometry
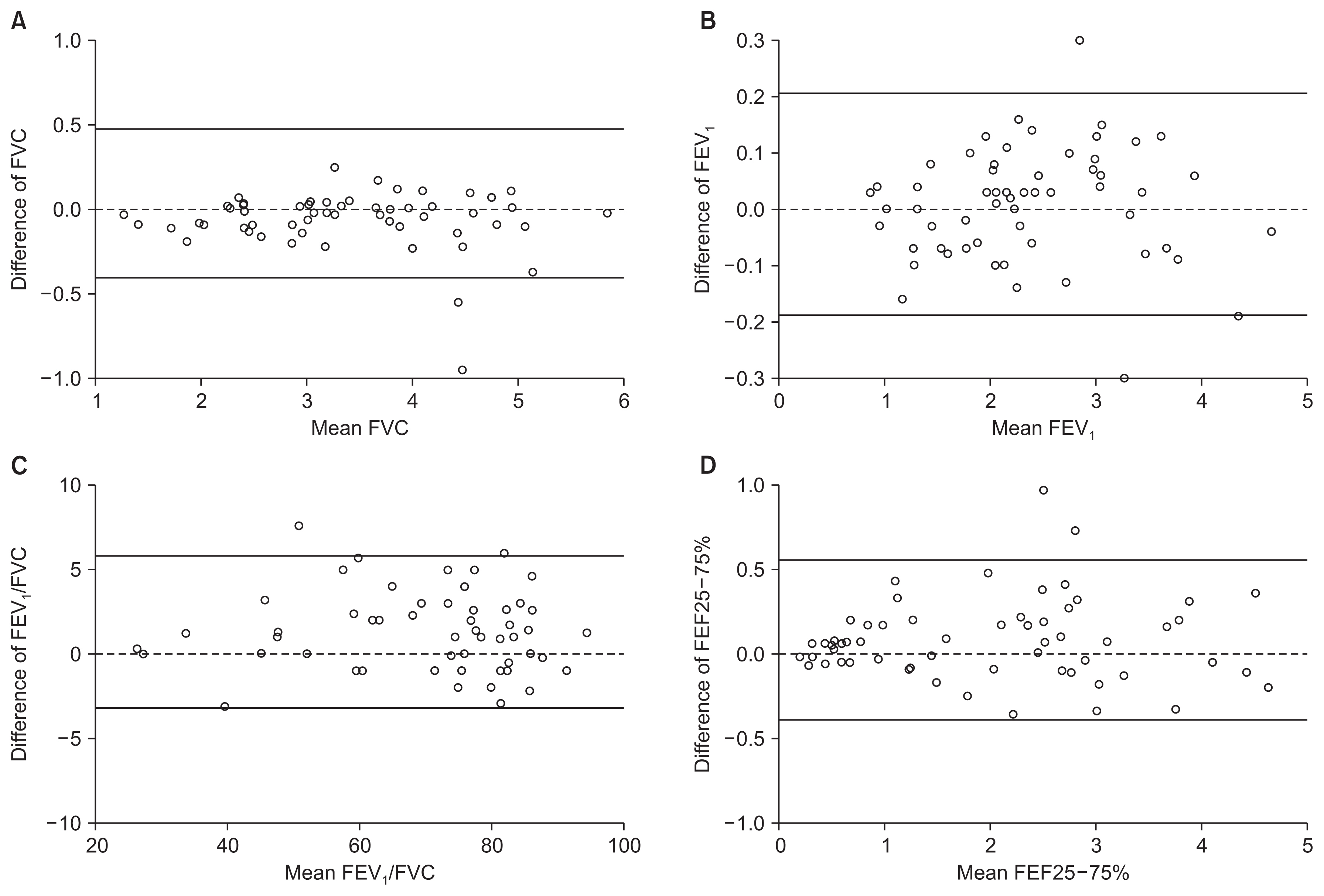
In the paired t-test, FVC estimated by portable spirometry (3.34 L) was slightly lower than that by conventional spirometry (3.40 L, p=0.009). However, the FEV1 values estimated by portable spirometry (2.36 L) and conventional spirometry (2.37 L) were not significantly different (p=0.516). The FEV1/ FVC ratio estimated by portable spirometry (71.5%) was slightly higher than that by conventional spirometry (70.2%, p<0.001). In addition, FEF25–75% ratio estimated by portable spirometry (2.03 L) was slightly higher than that by conventional spirometry (1.95, p=0.013) (Figure 2). Bland-Altman plot also showed good correlation of the values estimated between two instruments (Figure 3).

Comparison of FVC (A), FEV1 (B), the FEV1/FVC ratio (C), and FEF25–75% (D) conducted by portable spirometry and conventional spirometry. FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; FEF25–75%: forced expiratory flow at 25–75%.
Discussion
This study showed excellent correlation between lung function estimated by portable spirometry and conventional spirometry. The representative indicators for reproducibility and reliability of the new method compared to the standardized method, ICC, and Pearson’s correlation, were significant in all three important parameters of lung function in total and in the subgroup analysis. Although some values were significantly different in the paired t-test, the differences were not clinically significant. This study also indirectly supported and re-enforced reliability of previous clinical study which used lung function data of KNHANES.
Moreover, despite the correlation being generally excellent, the correlation of FEV1/FVC measured by ICC and Pearson’s correlation was not relatively ideal than that of FVC and FEV1. The FEV1/FVC is a critical indicator in diagnosing COPD. The presence of a post-bronchodilator FEV1/FVC<0.7 confirms the presence of persistent airflow limitation, which leads to the diagnosis of COPD. The range of FEV1/FVC is relatively small, so the FEV1/FVC is sensitive to various factors. The value may be changed because of biological variation15,16. Therefore, the recent national COPD guidelines suggest that the post-bronchodilator FEV1/FVC ratio be confirmed by repeated spirometry on a separate occasion if the value is between 0.6 and 0.817. We suggest that a modest correlation of the FEV1/ FVC estimated by portable and conventional spirometry can be explained by insignificant biological variation.
Here, the estimated value of FVC by portable spirometry was significantly lower than that by conventional spirometry. We speculated that this might be lead to relatively low sensitivity to detect extremely low expiratory flow in final exhalation by portable spirometry. The difference of the value was minimal at approximately 60 mL. Additionally, the FEV1/FVC measured by portable spirometry was significantly higher than that by conventional spirometry, although the difference was also minimal at approximately 1.3%. Although these minimal differences are not clinically significant, these differences could make the data on the prevalence of restrictive and obstructive diseases inaccurate. Hence, we should consider this significant difference when we interpret the clinical study results of lung function from the KNHANES data.
Recently, a handy spirometer has been developed18,19, although its compactness can be a disadvantage because frequent movement, crash, and adjustment can lead to several errors and breakdown of this vulnerable equipment. The conventional spirometer is big, non-movable, accurate, and solid. Thus, it is more preferred than the portable one in the clinical setting to obtain accurate information. However, some institutions cannot use conventional spirometry because of space, cost, and convenience. In the KNHANES, portable spirometry was used because this national study should be conducted in a moving bus that travels nationwide. However, studies have been insufficient to confirm the reliability of portable spirometry. We, for the first time, aimed to reveal the reproducibility and reliability of portable spirometry used in KNHANES data.
Our study results suggest that lung function measured by portable spirometry is highly correlated with that by conventional spirometry. If the physicians who use spirometry are highly trained specialists, then we can trust the results of the portable spirometry. In fact, the KNHANES employed specialists to perform the spirometry and have trained them regularly. Additionally, the spirometry results have been periodically verified. Therefore, we suggest that the spirometry results of lung function from the KNHANES are reliable regardless that the data was estimated by portable spirometry.
We attempted to reduce the study bias through the following protocol. First, we evenly included participants with various types of respiratory diseases. Second, we recruited participants from four distinct institutions. Third, we used three analytic methods (ICC, Pearson’s coefficient, and paired t-test) to define reliability20. Nonetheless, this study has some limitations. First, this study had a relatively small population, so further studies with a larger sample size will more strongly support our hypothesis. Second, we did not analyze post-bronchodilator lung function parameters. Third, we did not reveal the reliability of physicians who were engaged in the KNHANES. Thus, further extended studies will be helpful to re-confirm whether the lung function data of the KNHANES is reliable.
Lung function estimated by portable spirometry is well-correlated with that estimated by conventional spirometry. However, the values of FVC, FEV1/FVC, and FEF25–75% estimated using two types of spirometry have a small difference. We should consider this significant difference when we interpret the clinical study results of lung function contained in the KNHANES data. Generally, we suggest that the spirometry results contained in the KNHANES is reliable.
Notes
Authors’ Contributions
Conceptualization: Park YB. Methodology: Park HJ, Rhee CK, Yoo KH, Park YB. Software: Park HJ, Rhee CK, Yoo KH, Park YB. Validation: Park HJ, Rhee CK, Yoo KH, Park YB. Investigation: Park HJ, Rhee CK, Yoo KH, Park YB. Writing - original draft preparation: Park HJ. Writing - review and editing: Park HJ, Park YB. Approval of final manuscript: all authors.
Conflicts of Interest
Chin Kook Rhee serves as deputy editor of the Tuberculosis of Respiratory Diseases, but has no role in the decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (fund code: 2020-E3405-00).