 |
 |
| Tuberc Respir Dis > Volume 75(2); 2013 > Article |
|
Abstract
Background
More than half of cases for advanced non-small-cell lung cancer (NSCLC) occur in elderly patients with a median age at diagnosis of 70 years. The aim of our study was to examine the clinical features and prognostic factors contributing to mortality in elderly patients with advanced NSCLC.
Methods
Following a retrospective review of clinical data, 122 patients aged 70 years and over with a histopathological diagnosis of locally advanced (stage IIIB, n=32) and metastatic (stage IV, n=90) NSCLC between 2005 and 2011 were enrolled.
Results
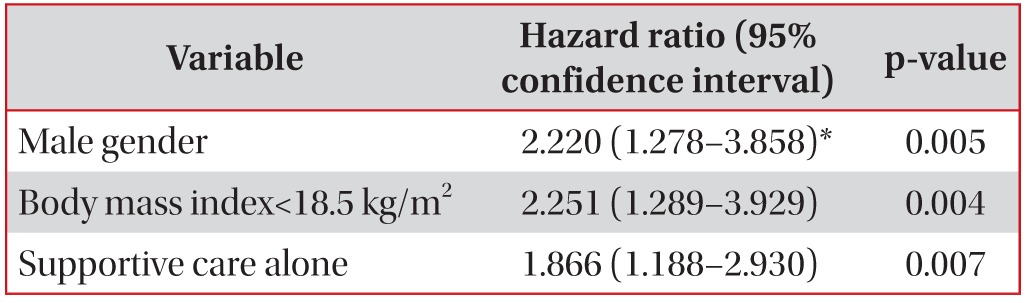
The median age was 76 years (interquartile range, [IQR], 72-80 years), and 85 (70%) patients were male. Fifty-seven (46%) patients had never smoked, and 17 (19%) were in a malnourished state with a body mass index (BMI) of <18.5 kg/m2. The initial treatments included chemotherapy (40%) and radiotherapy (7%), but 57% of the patients received supportive care only. The 1-year survival rate was 32%, and the 3-year survival rate was 4%, with a median survival duration of 6.2 months (IQR, 2.5-15.3 months). Male gender (hazard ratio [HR], 2.2; 95% confidence interval [CI], 1.3-3.9; p=0.005), low BMI (HR, 2.3; 95% CI, 1.3-3.9; p=0.004), and supportive care only (HR, 1.9; 95% CI, 1.2-2.9; p=0.007) were independent predictors of shorter survival based on a Cox proportional hazards model.
Compared with other cancers, the incidence of lung cancer has decreased since 2000. However, it remains the leading cause of cancer mortality according to Korean national statistics1. In addition, it has been reported that compared with other cancers, the incidence of lung cancer in individuals aged 70 years and older has increased rapidly1. According to a survey conducted by the Society of Korean Lung Cancer, the average age of patients initially diagnosed with lung cancer in 2005 was 64.7 years, and ~14% were over the age of 75 years2. Of the patients reviewed from a therapeutic perspective, 73.4% among all subjects underwent antitumor therapy, whereas only 47.1% in elderly subgroup more than 75 years of age received antitumor therapy2. The incidence of antitumor therapy in elderly subgroup was significantly less than those in the all subjects group.
More than 60% of patients diagnosed with non-small-cell lung cancer (NSCLC) have stage IIIB disease or higher3, and surgical treatment is not possible at the time of diagnosis. Although elderly patients required initial chemotherapy and/or radiotherapy, the treatment may be delayed secondary to family values in Korea; decisions may be made by members of the family and not from a medical standpoint4. In addition, physicians often do not perform aggressive treatment in elderly patients because of concerns regarding aging, organ dysfunction, and associated multiple comorbid conditions. However, an increased interest in appropriate antitumor therapy for elderly patients with lung cancer could improve survival5,6; thus, recognition of this issue is needed.
In a previous study, prognostic factors of NSCLC included age, gender, performance status (PS), histological type, stage, and treatment2. Advanced age was associated with a poor clinical outcome in patients with NSCLC. Much research has focused on the clinical outcomes and prognostic factors in patients with advanced NSCLC. Few studies have attempted to identify the clinical features and outcomes, and the predictive factors associated with mortality in elderly patients with advanced NSCLC are not well defined. This retrospective study was undertaken to identify the clinical features and prognostic factors contributing to mortality in elderly Korean patients with advanced NSCLC.
Patients with a diagnosis of primary lung cancer confirmed by cytologic or histologic evaluation, who were over the age of 70 years, and who were admitted to the Ewha Womans University Hospital between January 2005 and January 2011 were enrolled. Following a review of the medical records, data on age at the time of diagnosis, gender, smoking history, body mass index (BMI, kg/m2), comorbid conditions, pulmonary function, PS, histological type, treatment, and final clinical outcome were obtained. PS was based on the Eastern Cooperative Oncology Group (ECOG) classification. Histologically, separated NSCLC and small cell lung cancer (SCLC), SCLC were excluded from the study. Histological analysis of the tumor was based on the World Health Organization classification7. Using the clinical TNM classification, the study was limited to patients with stage IIIB or higher disease3. The patients who were transferred to other hospital for treatment or not followed up after diagnosis were excluded from this study subjects. Finally, 122 patients ware included. They were classified into groups according to treatment modality.
All patients underwent chest X-ray, chest computed tomography (CT), and evaluation of percutaneous needle aspiration samples, sputum cytology, and/or bronchoscopic biopsies. For diagnostic confirmation of metastasis to other sites, patients underwent bone scans, positron emission tomography, and brain magnetic resonance imaging or CT. Survival and causes of death were identified from the medical records, by interviews with the patients' families/doctors, or by accessing the national death registry data. The retrospective review of medical records and radiographic findings was approved by the Institutional Review Board of Ewha Medical Center (IRB No. 13-09-02).
For statistical analysis, the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used throughout the study, and p<0.05 was taken to indicate statistical significance. Continuous variables were summarized by calculating the median and interquartile range (IQR). Cumulative survival probabilities were estimated using the Kaplan-Meier method, and the log-rank test was applied to compare survival curves between patient characteristics. Cox proportional hazard multivariate analysis was performed to identify the independent factors closely related to death. Factors associated with mortality in the univariate analysis were entered into the multivariate model, and nonsignificant factors were removed by means of a backward-selection procedure. Hazard ratios (HR) with 95% confidence intervals (CI) were used to report the results.
The clinical characteristics of the 122 patients are listed in Table 1. The median age was 76 years (range, 72-80 years) with 85 (70%) male patients. There were 47 patients (39%) with an ECOG PS of Ōēź3. Nineteen percent of patients had a BMI of <18.5 kg/m2. Sixty-five patients (54%) had a history of smoking; 31 were current smokers and 34 were ex-smokers. Hypertension (34%) was the most common comorbidity, followed by chronic obstructive lung disease (24%), diabetes mellitus (16%), and pulmonary tuberculosis (13%). Pulmonary function test results were available for 82 patients (67%) at diagnosis and showed a median predicted forced vital capacity (FVC) of 77%, a predicted forced expiratory volume in 1 second (FEV1) of 77%, and an FEV1/FVC ratio of 69%. The patients were followed for a median of 4.2 months (IQR, 0.9-12.6 months).
Among all 122 patients, 90 (74%) were diagnosed at stage IV. In terms of the histologic distribution, squamous cell carcinoma (33.6%) was the most frequent histologic type, followed by adenocarcinoma (32.8%), non-small cell carcinoma (23.7%), and others. Stage IV was divided into M1a and M1b according to metastasis sites. Sixty-five patients (53%) were M1a, and 49 patients (40%) were M1b. The most common site of distant metastasis was bone (Table 2).
Sixty-nine (56.6%) patients were managed with symptomatic supportive care without antitumor therapy. Regarding the initial antitumor therapy, 49 patients received chemotherapy, and nine received radiotherapy (Table 3). The first-line chemotherapy in most patients comprised platinum-based combination therapy (n=31).
The overall clinical outcomes are summarized in Table 4. Among the 122 patients, 111 had died as of January 2011. The median survival time was 6.2 months (IQR, 2.5-15.3 months) for patients with advanced NSCLC. According to the stage, the median survival of patients with stage IIIB disease was 7.0 months (IQR, 3.3-14.5 months) and that of patients with stage IV disease was 6.0 months (IQR, 2.3-15.5 months). The 1-year survival rate was 32%, and the 3-year survival rate was 4%. The most common cause of death was lung cancer progression (Table 4). During the follow-up period, adverse clinical respiratory events occurred in 36 patients (29.5%); pneumonia (n=25) was the leading cause requiring a visit to the hospital, followed by hemoptysis (n=8) and pulmonary thromboembolism (n=3).
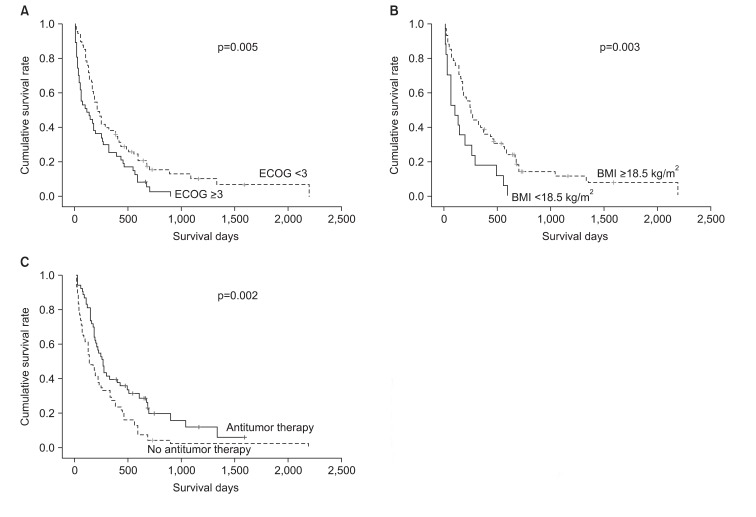
Three factors were associated with mortality based on the Kaplan-Meier survival curves and log-rank tests (p<0.05): an ECOG PS of Ōēź3 (p=0.005), a BMI of <18.5 kg/m2 (p=0.003), and symptomatic supportive care without antitumor therapy (p=0.002) (Figure 1). Patients with a poor ECOG PS of Ōēź3 showed a shorter median survival than those with an ECOG PS of Ōēż2 (123 days [95% CI, 7-238 days] vs. 221 days [95% CI, 150-292 days]; p=0.005). Patients with a BMI of <18.5 kg/m2 had a median survival of 103 days (95% CI, 35-171 days), while those with a BMI of Ōēź18.5 kg/m2 had a median survival of 252 days (95% CI, 148-356 days; p=0.003). The median survival for patients with any one of the antitumor therapies was 262 days (95% CI, 206-318 days), while that for patients who had received symptomatic supportive care without antitumor therapy was 143 days (95% CI, 84-202 days; p=0.002). However, no significant difference was found in median survival with respect to smoking history (p=0.293), stage (p=0.700), and histology type (p=0.116). After considering age, gender, smoking history, stage and histology, these three factors were introduced into the Cox regression hazard model using a backward-elevation method, which revealed that male gender (HR, 2.22; 95% CI, 1.28-3.86; p=0.005), low BMI (HR, 2.25; 95% CI, 1.29-3.93; p=0.004), and receiving supportive care only (HR, 1.87; 95% CI, 1.19-2.93; p=0.007) were independent predictive factors for a shorter survival duration (Table 5).
The results of the present study show that elderly patients with advanced NSCLC have a poor prognosis, particularly male patients, those with a low BMI, and those who receive symptomatic supportive care without antitumor therapy. Our results suggest that female patients, those with a good nutritional status, and those who have received antitumor therapy could be expected to have a better prognosis even in aged.
Since 2000, lung cancer in Korea as well as that worldwide has remained a leading cause of cancer death disease, and its incidence is expected to increase1,8. Previous studies have often been based on those over than 65 years of age, as a study including elderly population epidemiologically, but most recent studies of chemotherapy in elderly patients have been based on those over 70 years of age; thus, our study population was Ōēź70 years old. According to a Korean report, the most common histologic type of NSCLC is adenocarcinoma. However, Kim et al.9 surveyed one region in 2008 and found a higher incidence of squamous cell carcinoma than adenocarinoma; Jung et al.4 reported the same results in 2012. Likewise, our data suggest a higher incidence of squamous cell carcinoma (33.6%) than of adenocarcinoma (32.8%). These results were thought to be associated with age10,11 and gender2,11. The proportion of histologic types of cancer other than adenocarcinoma, such as squamous cell carcinoma, increased with age. Moreover, because this study included mostly male patients (70%), our results were expected to be similar. However, in this study the third most common histologic type was non-small-cell carcinoma. In an analysis of non-small cell carcinoma by year, no difference was found from 2005 to 2008; however, since 2009, the frequency of this histologic result has decreased substantially. This may be because of the increasing sophistication of histological classification due to developments in technology and immunology. Histological classification may have been underestimated in our study; additional research is needed to further clarify this issue.
Berghmans et al. reviewed conventional prognostic factors in stage III NSCLC by performing a meta-analysis12. According to this review, age, gender, PS, histology, and hemoglobin level were the most commonly reported prognostic factors in nonoperable stages of NSCLC12. Female patients with lung cancer have a longer survival period than do males13, as reported in a survey of patients in Korea2,11, female patients showed a better prognosis than those of male in our study. Similarly, the most common histologic type in female patients in the present study was adenocarcinoma (60%), and most female patients were nonsmokers (76%). Other factors, such as cancer stage and initial treatment, showed no differences between the genders. But Jang et al.11 suggested that gender differences have an influence until 70 years of age, but not beyond. So further studies involving elderly female patients with lung cancer are warranted.
Some studies have shown that the ECOG PS score is increased in elderly patients13 and is a prognostic factor for advanced NSCLC14. However, other studies have suggested no relationship between age and PS15. In this study, 39% of patients had a poor PS (ECOG PS score Ōēź3), and our findings suggest that such patients have a poorer prognosis, although this factor may not have been significantly associated with prognosis because of the small sample size. Our findings show that patients with a lower BMI have a poorer prognosis. BMI is known to reflect the general nutritional status, which is an important factor affecting the response to treatment and progression. The influence of BMI in several types of cancers has been investigated16. However, of lung cancer, BMI rarely has been studied. Yang et al.17 suggested that variations of patient's weight could be related with survival in lung cancer, and Luo et al.18 suggested low BMI to be a predictor of survival in patients with NSCLC.
More than half of the patients in the present study did not receive antitumor therapy and were managed with symptomatic supportive care. This result is similar to another survey of elderly patients in Korea2, in which antitumor therapy was an independent prognostic factor for lung cancer. Our findings also suggest that this factor affected survival, despite enrollment of only elderly patients. However, among 75 patients with a good PS (ECOG PS of Ōēż2), 40 patients did receive active antitumor therapy in this study. The authors therefore consider that the efforts of physicians would be required to change an awareness of antitumor therapy in elderly patients to both patients and caregivers. Undergoing an antitumor therapy increased the medial survival period, and thus affected survival. Given this evidence that treatment can prolong survival, physicians should advise elderly patients with advanced NSCLC accordingly. Because our study was enrolled patients before epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) being used as initial antitumor therapy in Korea, only a small number of patients were treated with EGFR-TKI. However, EGFR-TKI was known to be effective to particularly elderly and poor PS patients, for patients with either never-smoker status or adenocarcimona; histologic type19. NSCLC Patients should be evaluated to provide them with the most appropriate antitumor therapy based on tumor biology and be considered oral agents for initial antitumor therapy based on general status.
Our study has several drawbacks related to its retrospective nature and inclusion of a relatively small number of cases from only one tertiary hospital. Our inclusion of only patients with advanced NSCLC means that the subjects were not representative of the clinical characteristics of the entire population of elderly patients with NSCLC. More large-scale studies of elderly patients with NSCLC are warranted.
In conclusion, male gender, low BMI, and symptomatic supportive care without antitumor therapy were poor prognostic factors for survival. According to the current results and previous studies, elderly with advanced NSCLC including poor PS patients, benefit from antitumor therapy, although they tend to remain undertreated. In future, because lung cancer in elderly patients will increase and the medical environment will be developed at the same time, all advanced NSCLC patients should be referred for therapeutic evaluation, physicians should actively recommend to receive antitumor therapy by providing appropriate information about the lung cancer and understanding about the effects of treatment to patients and their families. And additional molecular evaluation may offer to match patients with available treatment. Therefore, although elderly advanced NSCLC patients, antitumor therapy is positively considered because those affected survival.
Acknowledgements
We thank Kyung Ae Kong, M.D., Ph.D. (Ewha Medical Center and Ewha Medical Research Institute, Ewha Womans University School of Medicine, Seoul, South Korea) for assistance with the statistical analysis.
References
1. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011;43:1-11. PMID: 21509157.




2. Kim YC, Kwon YS, Oh IJ, Kim KS, Kim SY, Ryu JS, et al. National survey of lung cancer in Korea, 2005. J Lung Cancer 2007;6:67-73.

3. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-714. PMID: 17762336.


4. Jung SY, Park DI, Park MR, Jung SS, Kim JO, Kim SY, et al. Clinical characteristics of patients older than 76 with lung cancer. Korean J Med 2012;82:562-568.


5. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003;95:362-372. PMID: 12618501.



6. Schild SE, Stella PJ, Geyer SM, Bonner JA, McGinnis WL, Mailliard JA, et al. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol 2003;21:3201-3206. PMID: 12874270.


7. The World Health Organization histological typing of lung tumours. Second edition. Am J Clin Pathol 1982;77:123-136. PMID: 7064914.


8. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. PMID: 22237781.


9. Kim HS, Hyun DS, Kim KC, Lee SC, Jung TH, Park JY, et al. The clinical characteristics and prognosis of elderly patient with lung cancer diagnosed in Daegu and Gyeongsangbukdo. Tuberc Respir Dis 2008;65:15-22.

10. Hsu CL, Chen KY, Shih JY, Ho CC, Yang CH, Yu CJ, et al. Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer 2012;12:241PMID: 22695392.




11. Jang TW, Kim YC, Kwon YS, Oh IJ, Kim KS, Kim SY, et al. Female lung cancer: re-analysis of national survey of lung cancer in Korea, 2005. J Lung Cancer 2010;9:57-63.

12. Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol 2011;3:127-138. PMID: 21904576.



14. Brown JS, Eraut D, Trask C, Davison AG. Age and the treatment of lung cancer. Thorax 1996;51:564-568. PMID: 8693434.



15. Montella M, Gridelli C, Crispo A, Scognamiglio F, Ruffolo P, Gatani T, et al. Has lung cancer in the elderly different characteristics at presentation? Oncol Rep 2002;9:1093-1096. PMID: 12168079.


16. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 2005;9(Suppl 2):S51-S63. PMID: 16437758.


17. Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res 2011;170:e75-e83. PMID: 21704331.



18. Luo J, Chen YJ, Narsavage GL, Ducatman A. Predictors of survival in patients with non-small cell lung cancer. Oncol Nurs Forum 2012;39:609-616. PMID: 23107855.



19. Langer CJ. Clinical evidence on the undertreatment of older and poor performance patients who have advanced non-small-cell lung cancer: is there a role for targeted therapy in these cohorts? Clin Lung Cancer 2011;12:272-279. PMID: 21704567.


Figure┬Ā1
Kaplan-Meier Survival curves according to significant prognostic factors. (A) The cumulative survival rate as depicted graphically by the Kaplan-Meier method; p=0.005, ECOG Ōēź3 vs. ECOG <3. (B) The cumulative survival rate as depicted graphically by the Kaplan-Meier method; p=0.003, BMI <18.5 kg/m2 vs. BMI Ōēź18.5 kmg/m2. (C) The cumulative survival rate as depicted graphically by the Kaplan-Meier method; p=0.002, antitumor therapy vs. no antitumor therapy.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




