 |
 |
| Tuberc Respir Dis > Volume 75(4); 2013 > Article |
|
Abstract
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is rare, with a more favorable prognosis compared with that of other types of non-small cell lung cancers. Herein, we describe an interesting case of primary pulmonary LELC confirmed postoperatively, which had been initially diagnosed as poorly differentiated adenocarcinoma. We suggest that despite the rarity of pulmonary LELC, it should be included as one of the differential diagnoses for lung malignancies. Physicians should consider taking a larger biopsy, especially when histologic examination shows undifferentiated nature.
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) was first reported in 19871. Although there are over 150 cases of English literature highlighted primary pulmonary LELC as one of non-small cell lung cancers (NSCLCs), it occupies only small portion of the lung cancer2,3. It displays a unique process of carcinogenesis, which is related with Epstein-Barr virus (EBV) infection and possesses similar histologic features to those of nasopharyngeal carcinoma4. In addition, it has been known to result in more favorable prognosis than other types of NSCLCs5. Considering these differences, it is important to distinguish primary pulmonary LELC from other types of NSCLCs.
Herein, we describe an interesting case confirmed as primary pulmonary LELC postoperatively, which had initially been regarded as undifferentiated adenocarcinoma by histologic analysis with the tissue sample obtained through percutaneous transthoracic needle biopsy (PTNB).
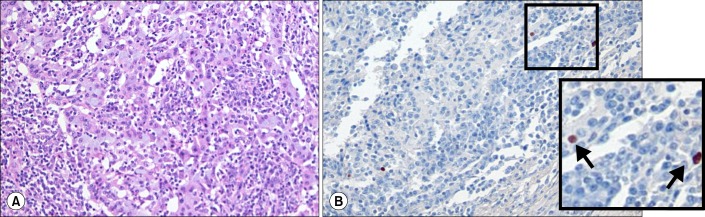
A 60-year-old woman visited our outpatient clinic for evaluation of a lung mass incidentally detected on chest computed tomography (CT) during regular medical check-up. Physical and laboratory examination did not reveal any relevant abnormalities. She had no medical illness and was never-smoked. Initial chest radiograph revealed a nodular opacity in the right middle lung field (Figure 1A). On chest CT, there was an approximately 3.1 cm mass in diameter with spiculated margin and heterogeneous enhancement in the right lower lobe of the lung, which invaded right major fissure. In addition, there were adjacent ground-grass opacities posterior to the mass (Figure 1B-D). Next, we performed PTNB for the histological diagnosis for the mass. Microscopic examination showed that tumor cells were arranged in diffuse pattern and reveal round nuclei without prominent nucleoli and moderate amount of cytoplasm. The tumor cells were diffusely stained with p53, thyroid transcription factor-1, and Ki-67 on immunohistochemical analysis, suggesting that it was a malignant mass such as poorly differentiated adenocarcinoma. As for the staging work-up, positron emission tomography-CT and brain magnetic resonance revealed that the mass was hypermetabolic (standardized uptake value, 9.32) with high uptake of fluorodeoxyglucose (Figure 1E) and that there was no metastatic foci or lymphadenopathies, altogether, it was diagnosed as adenocarcinoma of cT2aN0M0 (stage IB) by TNM staging system. For her treatment, she underwent the right lower lobectomy. On histological examination, the tumor was grossly 1.8×1.5 cm-sized and ashen gray-colored mass with ill-defined margin. The mass was firm to palpation without pleural, vascular, and lymphatic invasion. It was composed of large epithelioid malignant cells with undifferentiated appearance, syncytial growth, and adjacent reactive lymphoplasmacytic cells influx (Figure 2A). Moreover, some of the malignant cells were positive for in situ hybridization with EBV-coded small RNA (EBER) (Figure 2B). Any glandular and squamous cell differentiation was not detected. Based on these histologic findings, the mass was finally diagnosed as primary pulmonary LELC of pT1aN0M0 (stage IA). After the surgical resection, there had been no recurrence or complication of disease at follow-up of 3 years.
Primary pulmonary LELC appears to have more favorable prognosis compared with those of other types of NSCLCs. A very recent study with 52 primary pulmonary LELC patients has demonstrated that the 2-year and 5-year overall survival rates were 88% and 62%, respectively, with most patients diagnosed at early or locally advanced stages6. This study has also indicated that patients with locally advanced disease achieve longer survival rate with multiple therapeutic modalities including surgery, chemotherapy, radiotherapy, and targeted therapy than that of patients without treatment6.
Nevertheless, histologic diagnosis of primary pulmonary LELC is difficult due to poor clinical suspicion by physicians, because of its scarcity with an estimated prevalence of about 1% of lung cancer in Southeast Asia, and much lower incidence in Western population2. Moreover, typical primary pulmonary LELC consists of undifferentiated carcinoma cells with ill-defined cytoplasmic borders arranged in syncytial sheets and stroma showing thick fibrous bands containing large numbers of reactive lympho-plasmacytic cells as well as other inflammatory cells2. Therefore, primary pulmonary LELC is not familiar to most physicians and may be interpreted as undifferentiated NSCLC by pathologists.
Although many guidelines accept the pathologic examination of cytologic or tissue-based preparations of the suspicious pulmonary malignant lesions as confirmative diagnostic methods, large biopsies, i.e., tissue-based biopsy through excision or surgical resection, at least for pulmonary LELC, may provide us more accurate histologic information than cytology or small biopsies via needle biopsy. For example, there are a number of disorders such as poorly differentiated carcinoma, malignant melanoma, and malignant lymphoma in which cytopathologic features resemble primary pulmonary LELC7. Interestingly, in our current case, the pathologic examination of the tissues resected surgically provided the correct diagnosis of primary pulmonary LELC, while the pathologic diagnosis based on the tissue sample obtained via PTNB was undifferentiated adenocarcinoma.
Moreover, in particular for a difficult case of pulmonary LELC in distinguishing from other lung malignancies, the detection of EBV infection may be helpful. Although, in some cases, EBV is not necessary for the development of LELC, many patients are positive for EBV, especially in its prevalent area. Among various diagnostic methods for the detection of EBV infection, in situ hybridization for EBER is the most specific and highly sensitive method6.
In this report, we describe a patient confirmed as having a primary pulmonary LELC postoperatively, which had initially been regarded as undifferentiated adenocarcinoma on tissue-based pathologic examination via PTNB. This clinical experience provide us with evidence supporting that for the confirmative diagnosis of primary pulmonary LELC, large biopsies, i.e., tissue-based biopsy through excision or surgical resection give us more accurate histologic information than cytology or small biopsies via needle biopsy.
Acknowledgements
We thank Professor Mie-Jae Im for critical readings of the manuscript. This work was supported by grants of the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A084144 to Yong Chul Lee and A111992 to So Ri Kim).
References
1. Begin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-283. PMID: 2826922.


2. Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539-545. PMID: 16916325.


3. Jung CY, Shim SW, Park CK, Kwon KY, Jeon YJ. A case of lymphoepithelioma-like carcinoma of the lung. Tuberc Respir Dis 2011;71:363-367.

5. Huang CJ, Feng AC, Fang YF, Ku WH, Chu NM, Yu CT, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012;13:359-362. PMID: 22410385.


6. Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao H, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-4758. PMID: 22359203.


7. Hayashi T, Haba R, Tanizawa J, Katsuki N, Kadota K, Miyai Y, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagn Cytopathol 2012;40:820-825. PMID: 21433005.


Figure 1
(A) Initial chest radiograph reveals approximately 2.5 cm-sized nodular opacity in the right middle lung field. (B.D) Chest computed tomography (CT) at admission. Coronal image (post-enhancement) (B), lung setting (C), and axial image (post-enhancement) (D), show a 3 cm-sized mass with spiculated margin, heterogeneous enhancement with ground glass opacities posterioly. (E) Positron emission tomography-CT shows hypermetabolic nodule (standardized uptake value, 9.32) of irregular shape in the right lower lobe.
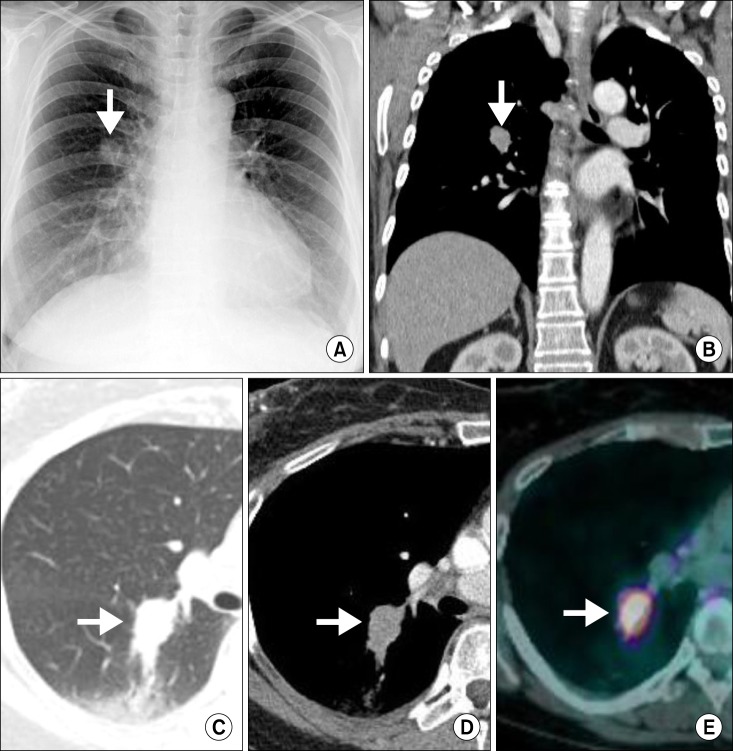
Figure 2
(A) There are many undifferentiated, large epithelioid malignant cells with indistinct cell borders (syncytial growth). A striking influx of lymphocytes around malignant cells is evident (H&E stain, ×200). (B) Some of the malignant cells are positive for in situ hybridization of Ebstein-Barr virus (EBV)-coded small RNA (×200). The right lower corner box (twice enlarged photo from the original image) shows the magnification view of positive reaction with EBV.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




