 |
 |
| Tuberc Respir Dis > Volume 77(1); 2014 > Article |
|
Abstract
Background
This study analyzed the negative prognostic factors in patients who received second-line chemotherapy for advanced inoperable non-small cell lung cancer (NSCLC).
Methods
We retrospectively reviewed the records of 137 patients with inoperable stage III-IV NSCLC who received second-line chemotherapy. The effects of clinical parameters on survival were analyzed and the hazard ratios (HR) for mortality were identified by a Cox regression analysis.
Results
Sex, age older than 65 years, smoking history, cell type, T-stage, best response to first-line chemotherapy and first-line chemotherapy regimen were significant negative predictors in univariate analysis. The multivariate analysis showed that patients older than 65 years (HR, 1.530; 95% confidence interval [CI], 1.020-2.297), advanced T stage (T4 vs. T1; HR, 2.273; 95% CI, 1.010-5.114) and non-responders who showed progression with first-line chemotherapy (HR, 1.530; 95% CI, 1.063-2.203) had higher HR for death.
Two thirds of lung cancers are diagnosed in late or advanced stages, leading to high mortality rates with unfavorable prognoses 1. Although chemotherapy for advanced lung cancer is known to improve survival and quality of life compared with symptomatic treatment, lung cancers usually still progress after chemotherapy and are often aggravated by treatment-related complications2,3. Second-line chemotherapy could be carried out by altering other regimens in patients who stop first-line chemotherapy, allowing a greater survival rate compared with patients given symptomatic treatment only4,5. However, extension of overall survival (OS) is not guaranteed for all patients; some patients suffer from reduced survival or are overwhelmed by side effects of chemotherapy. Clinical predictors of the relative advantages of chemotherapy and likelihood of complications could reduce unnecessary medical expenses and prevent unexpected deaths. Although the prognostic determinants of first-line chemotherapy have been widely studied, relatively few studies have been conducted on prognostic determinants of second-line chemotherapy. Reports on clinical factors that affect OS in patients who undergo second-line chemotherapy do not clearly agree6,7,8,9,10. Here, we analyzed clinical determinants of survival in second-line chemotherapy.
This study retrospectively reviewed patients diagnosed with stage III-IV non-small cell lung cancer (NSCLC) who received second-line chemotherapy from 2000 to 2009 in Ewha University Mokdong Hospital. The study was conducted after gaining institutional review board approval (ECT 12-38A-07). We reviewed subjects' records, including sex, age, body mass index, smoking, lactate dehydrogenase, albumin, hemoglobin, pathology, clinical stage, radiation treatment in chest, regimens and best response to first-line chemotherapy, adverse effect of first-line chemotherapy, regimens of second-line chemotherapy, and other baseline demographic parameters. Survival period was defined from the date of starting second-line chemotherapy to the date of death or last hospital visit before May 31, 2012, whichever came first.
First-line chemotherapy was based on National Comprehensive Cancer Network guidelines. Patients who progressed during first-line chemotherapy or some period after completing first-line chemotherapy, or who stopped chemotherapy because of adverse effects underwent second-line chemotherapy. Second-line chemotherapy was changed to a cytotoxic duplet regimen, or a cytotoxic single agent parenterally or oral tyrosine kinase inhibitor with the consideration of patients' clinical conditions and tumor status in previous first-line chemotherapy. Response to chemotherapy was evaluated by the Response Evaluation Criteria in Solid Tumor (RECIST) guidelines version 1.111.
A Cox regression was used to analyze hazard ratio of death for each variables. Multivariate analysis was performed with significant variables from the above analysis and estimated risk factors. p<0.05 was considered statistically significant. The SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
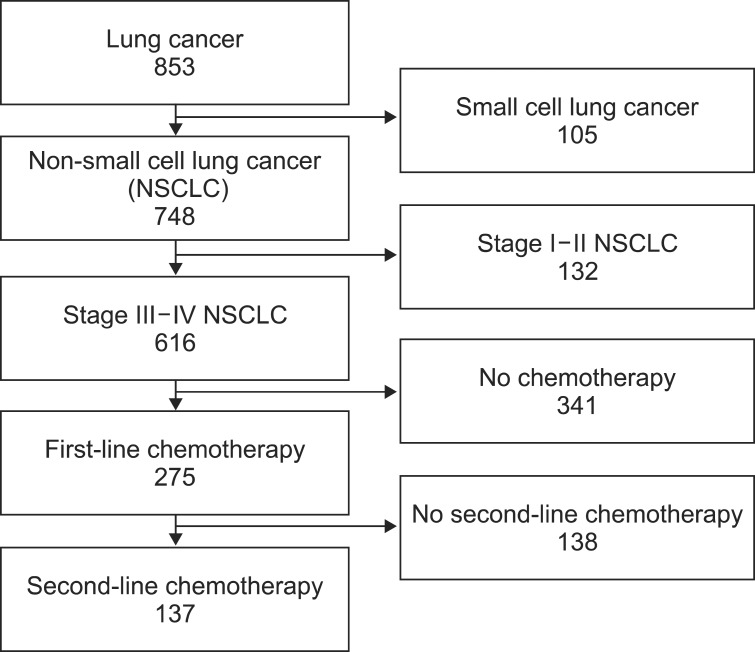
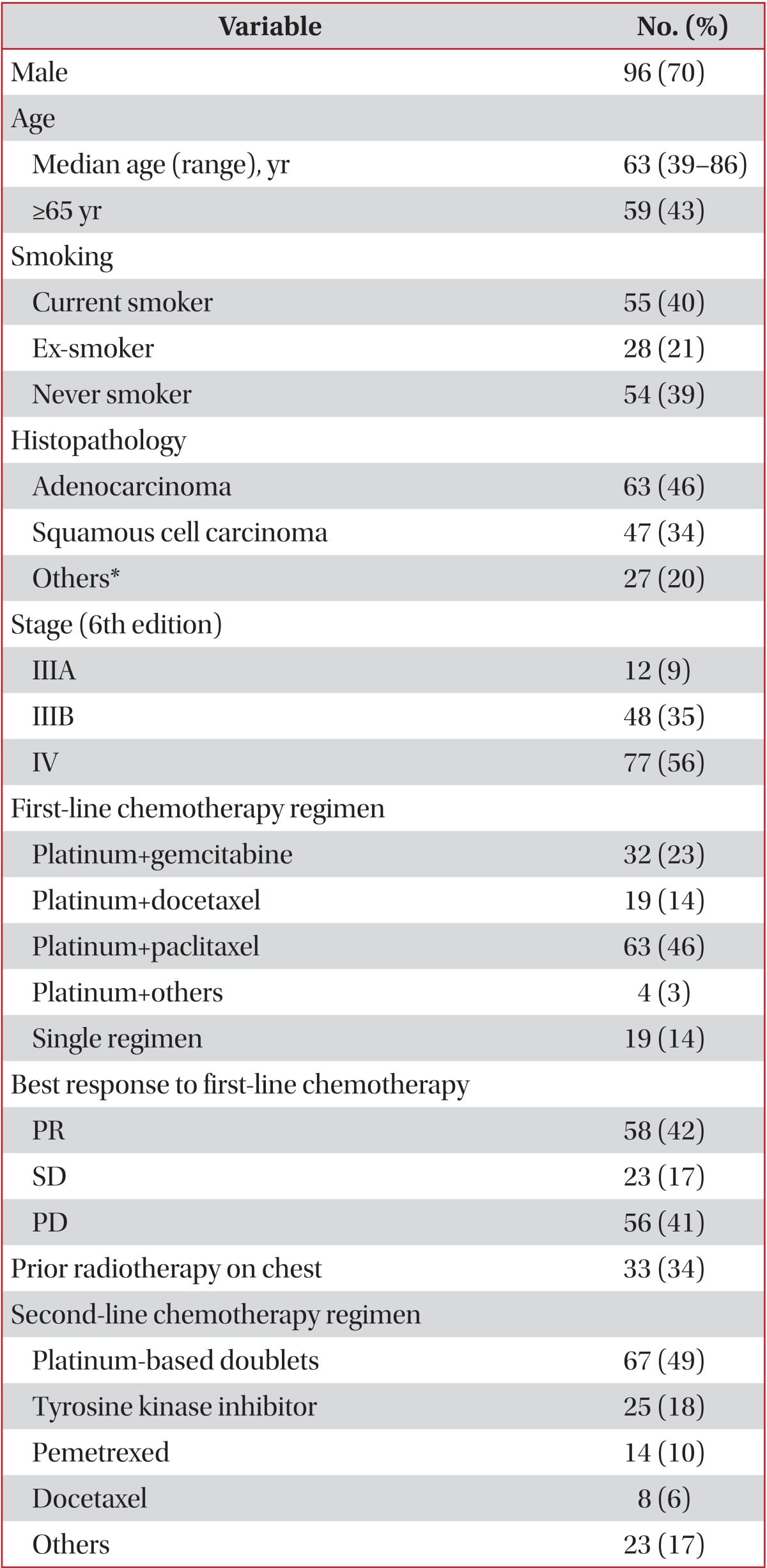
Of total 853 patients confirmed with lung cancer, 748 patients were diagnosed with NSCLC. First-line chemotherapy was undergone by 275 patients with stage III-IV, pathologically proven NSCLC who were inoperable or who had rejected surgery. Second-line chemotherapy was undergone by 137 patients who showed progression during first-line chemotherapy (n=98), or some time after completing first-line chemotherapy (n=7), or who stop receiving chemotherapy because of adverse effects, performance, or their own decision (n=32) (Figure 1). The median age was 63 years old (range, 39-86 years); 96 (70%) were male and 41 (30%) were female. Adenocarcinoma was the most common cell type (46%), followed by squamous cell carcinoma (34%). Best response to first-line chemotherapy was partial remission (PR) in 42% of patients, stable disease (SD) in 17%, and progressive disease (PD) in 41%. Platinum-based chemotherapeutics were used in 49% of patients for second-line chemotherapy. Tyrosine kinase inhibitors, pemetrexed, and docetaxel were prescribed as single agents (Table 1).
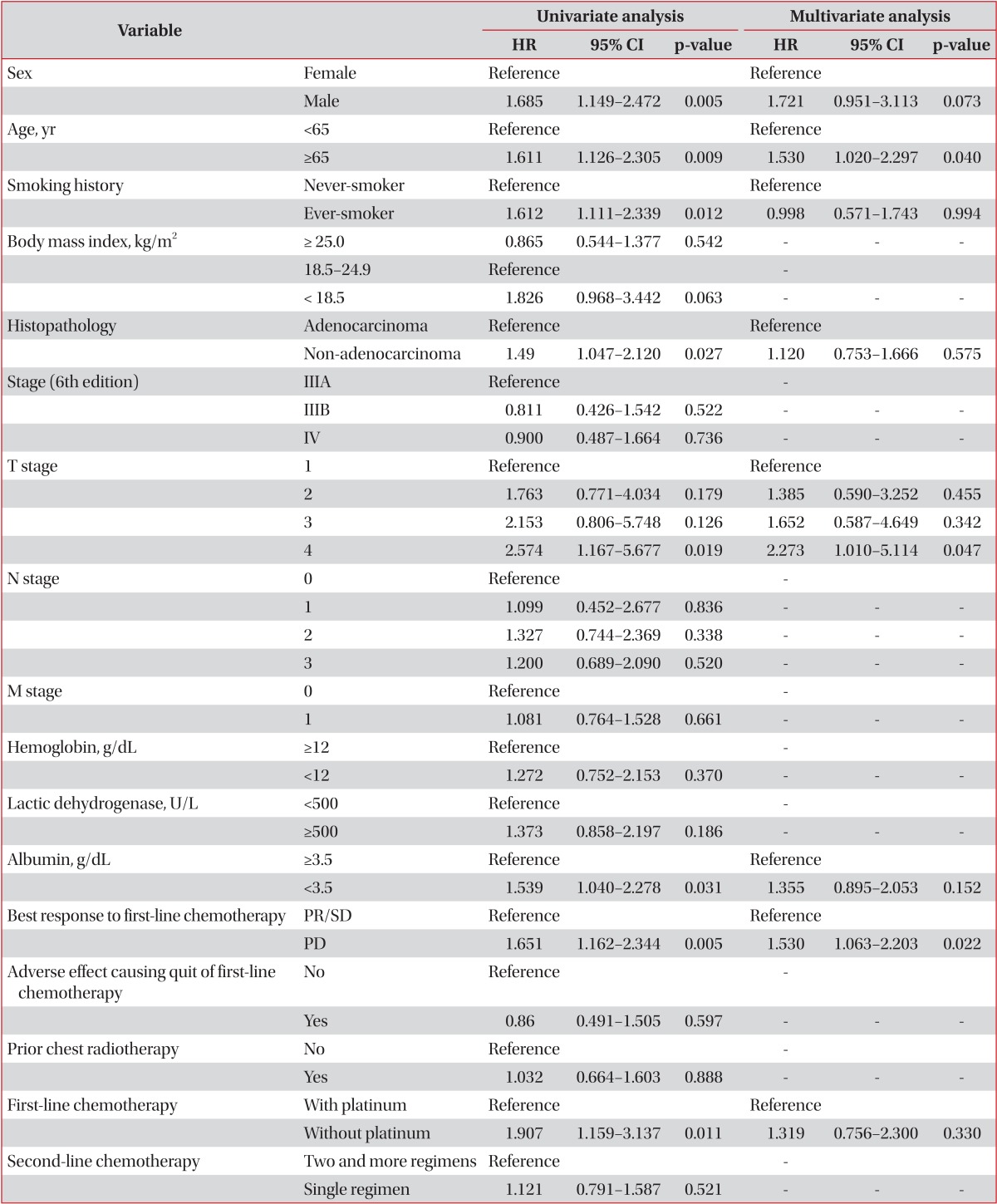
When we analyzed clinical indicators that affect survival, significant variables were sex, age>65 years, smoking, pathological classification, T-stage, albumin level, and best response to first-line chemotherapy in univariate analysis (Table 2). Cox regression was analyzed on significant prognostic determinants of the primary analysis. Patients aged 65 or older had 1.53 times higher risk of death than those younger than 65 years (confidence interval [CI], 1.02-2.30; p=0.040). Moreover, patients whose best response to first-line chemotherapy was PD showed 1.53 times higher risk of death than the SD and PR groups (CI, 1.06-2.20; p=0.022). In terms of T stage, stage T4 presented 2.27 times higher risk than stage T1 (CI, 1.01-5.11; p=0.047).
Age over 65 years, T4 tumor stage, and poor response to first-line chemotherapy were the most influential predictors of survival after second-line chemotherapy in this study. Second-line chemotherapy is performed in cases of PD during first-line chemotherapy or during follow-up period after completing first-line chemotherapy. Although second-line chemotherapy is implemented to improve the survival rate of cancer patients2,5, it can raise the hazard ratio in high risk groups8. The indication of second-line chemotherapy and selection of proper chemotherapeutic should be clearly defined, which necessitates identification of factors that predict the outcome of second-line chemotherapy. Earlier studies reported poor performance status, advanced clinical stage, longer intervals between completion of first-line chemotherapy and initiation of second-line chemotherapy, poor response during first-line chemotherapy, male sex, and histological type of non-adenocarcinoma as significant prognostic determinants6,7,8,9.
Our finding that outcomes of second-line chemotherapy rely on response to first-line chemotherapy aligned with results of previous studies7,8,10,12. Di Maio et al.7,10 reported that among patients with advanced NSCLC who undergo second-line chemotherapy, the hazard ratio of death in patients who did not respond to first-line chemotherapy was 1.25, similar to the 1.53 in the non-responding group in our study. Response to first-line chemotherapy seems to be an important predictor of response to second-line chemotherapy.
Unlike previous studies, we found higher risk of death in patients aged 65 or older than in patients aged younger than 65 years. Studies that compared prognosis between younger and elderly patients found no significant differences in response, time to progression, and OS in elderly patients (i.e., older than 70 years) compared with younger patients13,14. The discrepancy between our results and the earlier reports might be from differences in chemotherapy regimens and clinical setting. Elderly patients usually show more hematologic adverse effects during cytotoxic chemotherapy14.
Initial clinical stage affects the success of second-line chemotherapy6,7,9. Our multivariate analysis showed clinical T4 stage to be a significant risk factor compared with T1. Initial tumor stage appears to be a more important predictor of response to second-line chemotherapy than nodal stage or presence of metastasis in advanced lung cancer. Previous reports found that combination chemotherapy in second-line chemotherapy did not increase patients' survival rate, which accords with our results15,16,17.
This study had some limitations. It was a single-center retrospective study with a small sample size. During the enrollment period, treatment models and approaches to chemotherapy selection changed, especially with the introduction of molecular target agents. Although this study did not reflect these recent changes, it was analyzed in the light of mainly cytotoxic second-line agents. Additionally, this study did not include performance status at the time of second-line chemotherapy because of incomplete records.
Conclusively, age younger than 65 years, early T stage and good therapeutic responsive to initial chemotherapy predict favorable outcome of second-line chemotherapy. However, determinants of response to salvage chemotherapies should be further analyzed in a multicenter study.
Acknowledgements
There was no financial support. We thank Kyoung Ae Kong, M.D., Ph.D. for her statistical support.
References
2. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-2103. PMID: 10811675.


3. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-909. PMID: 7580546.



4. Tassinari D, Scarpi E, Sartori S, Tamburini E, Santelmo C, Tombesi P, et al. Second-line treatments in non-small cell lung cancer. A systematic review of literature and metaanalysis of randomized clinical trials. Chest 2009;135:1596-1609. PMID: 19225067.


5. Barlesi F, Jacot W, Astoul P, Pujol JL. Second-line treatment for advanced non-small cell lung cancer: a systematic review. Lung Cancer 2006;51:159-172. PMID: 16360238.


6. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-1597. PMID: 15117980.


7. Di Maio M, Lama N, Morabito A, Smit EF, Georgoulias V, Takeda K, et al. Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy: a prognostic score from individual data of nine randomised trials. Eur J Cancer 2010;46:735-743. PMID: 20045311.


8. Florescu M, Hasan B, Seymour L, Ding K, Shepherd FA. National Cancer Institute of Canada Clinical Trials Group. A clinical prognostic index for patients treated with erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR.21. J Thorac Oncol 2008;3:590-598. PMID: 18520796.


9. Weiss GJ, Rosell R, Fossella F, Perry M, Stahel R, Barata F, et al. The impact of induction chemotherapy on the outcome of second-line therapy with pemetrexed or docetaxel in patients with advanced non-small-cell lung cancer. Ann Oncol 2007;18:453-460. PMID: 17322539.



10. Di Maio M, Krzakowski M, Fougeray R, Kowalski DM, Gridelli C. Prognostic score for second-line chemotherapy of advanced non-small-cell lung cancer: external validation in a phase III trial comparing vinflunine with docetaxel. Lung Cancer 2012;77:116-120. PMID: 22361218.


11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247. PMID: 19097774.


12. Inal A, Kaplan MA, Kucukoner M, Urakci Z, Karakus A, Isikdogan A. Prognostic factors for second-line treatment of advanced non-small-cell lung cancer: retrospective analysis at a single institution. Asian Pac J Cancer Prev 2012;13:1281-1284. PMID: 22799319.



13. Weiss GJ, Langer C, Rosell R, Hanna N, Shepherd F, Einhorn LH, et al. Elderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2006;24:4405-4411. PMID: 16983108.


14. Wu CH, Fan WC, Chen YM, Chou KT, Shih JF, Tsai CM, et al. Second-line therapy for elderly patients with non-small cell lung cancer who failed previous chemotherapy is as effective as for younger patients. J Thorac Oncol 2010;5:376-379. PMID: 20104191.


15. Takeda K, Negoro S, Tamura T, Nishiwaki Y, Kudoh S, Yokota S, et al. Phase III trial of docetaxel plus gemcitabine versus docetaxel in second-line treatment for non-small-cell lung cancer: results of a Japan Clinical Oncology Group trial (JCOG0104). Ann Oncol 2009;20:835-841. PMID: 19164456.



16. Wachters FM, Groen HJ, Biesma B, Schramel FM, Postmus PE, Stigt JA, et al. A randomised phase II trial of docetaxel vs docetaxel and irinotecan in patients with stage IIIb-IV non-small-cell lung cancer who failed first-line treatment. Br J Cancer 2005;92:15-20. PMID: 15597104.



17. Pectasides D, Pectasides M, Farmakis D, Kostopoulou V, Nikolaou M, Gaglia A, et al. Comparison of docetaxel and docetaxel-irinotecan combination as second-line chemotherapy in advanced non-small-cell lung cancer: a randomized phase II trial. Ann Oncol 2005;16:294-299. PMID: 15668287.



- TOOLS
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 7,115 View
- 24 Download
- Related articles
-
Recent Advances in Adjuvant Therapy for Non–Small-Cell Lung Cancer2024 January;87(1)
New Targeted Therapy for Non-Small Cell Lung Cancer2023 January;86(1)
High VPP combination chemotherapy for advanced non-small cell lung cancer.1993 August;40(4)


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



