 |
 |
| Tuberc Respir Dis > Volume 77(1); 2014 > Article |
|
Abstract
Takotsubo cardiomyopathy (TTC) is defined as a reversible, acute ventricular dysfunction without any evidence of coronary artery obstruction. There have been reports of TTC caused by emotional or physical stress, drug use, hormone imbalance, or medical conditions such as pulmonary disease, sepsis, and trauma, but a relationship between TTC and pulmonary tuberculosis has not previously been reported. From our knowledge, this is the first report of TTC caused by pulmonary tuberculosis.
Takotsubo cardiomyopathy (TTC) is an acute syndrome characterized by reversible ventricular dysfunction in the absence of significant coronary artery disease1. Several inconsistent hypotheses have been proposed regarding the pathophysiology of TTC. Currently, catecholaminergic overstimulation caused by mental and/or physical stress is accepted as a reasonable pathophysiology of TTC2. However, the etiology and intensity of how stress causes TTC are still unclear. In this case, we suggest that pulmonary tuberculosis may be a precipitant.
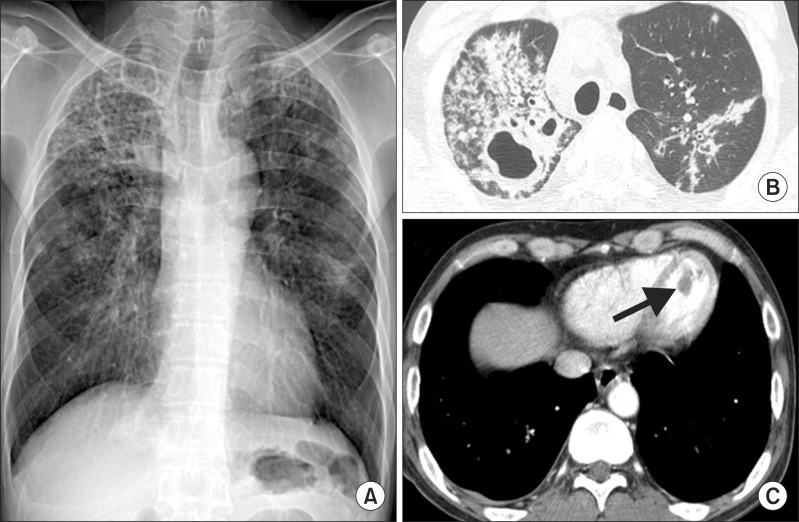
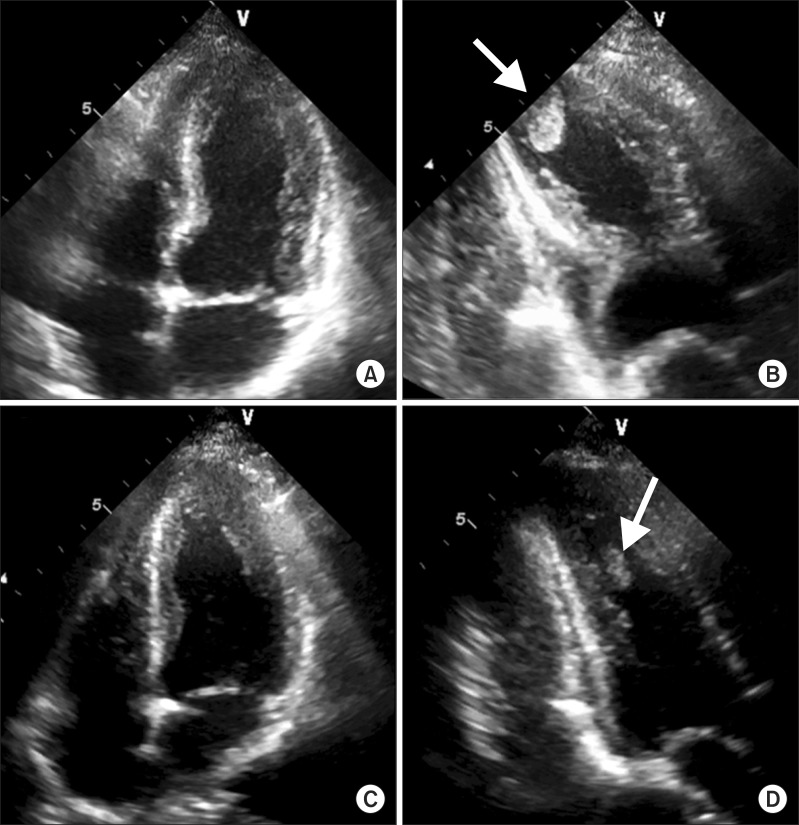
A 54-year-old Korean man presented with a month-long history of cough and yellowish sputum, an 8-kg weight loss over the course of three months and Modified Medical Research Council Dyspnea Scale grade 1 dyspnea. He had no history of surgery or other medical illness and he was a current smoker (30 pack-years). On examination, his blood pressure was 100/80 mm Hg, pulse rate was 76 beats/min, respiratory rate was 24 breaths/min, and body temperature was 36.5Ōäā. On chest auscultation, breathing sounds were abnormal with coarse rales in both upper lung fields. Initial arterial blood gas results were pH 7.428, pCO2 32.8 mm Hg, pO2 85.1 mm Hg, HCO3- 21.2 mmol/L, and SaO2, 97.8% in ambient air. Blood tests showed a white blood cell count of 6,700/mm3 (normal, 4000-10000/mm3), hemoglobin of 12.8 g/dL (normal, 12.5-15 g/dL), and a platelet count of 178/mm3 (normal, 150-450/mm3). The C-reactive protein level was found to be 5.23 mg/L (normal, 0-0.75 mg/L). A blood chemistry panel revealed a blood urea nitrogen level of 45.3 mg/dL (normal, 7-20 mg/dL), creatinine of 1.0 mg/dL (normal, 0.5-1.5 mg/dL), total protein of 8.3 g/dL (normal, 6.0-8.3 g/dL), albumin of 3.2 g/dL (normal, 3.5-4.5 mg/dL), and aspartate aminotransferase/alanine aminotransferase of 78/54 IU/L (normal, 8-40/5-35, respectively). Cardiac enzyme labs were found to be elevated: CK-MB, 33.21 ng/mL (normal, 0-5 ng/mL); troponin-I, 0.904 ng/mL (normal, 0.0-0.2 ng/mL); and brain natriuretic peptide, 183.20 pg/mL (normal, 0-99 pg/mL). Electrocardiography (ECG) revealed nonspecific ST- or T-wave abnormalities. Initial chest radiographs showed numerous nodules in both apices suggestive of active pulmonary tuberculosis (Figure 1A). Chest computed tomography (CT) showed two large cavities in the right upper lobe and small nodules in both lungs (Figure 1B). There were no abnormal lesions present in either adrenal gland. There was a 1.9-cm ovoid filling defect in the apex of the left ventricle (LV) suggestive of a thrombus (Figure 1C). Echocardiography showed a balloon-like akinesia of the apex wall (Figure 2A), a 1.65├Ś1.21-cm apical thrombus (Figure 2B), and the left ventricular ejection fraction was 56.7%.
We started anticoagulation with low molecular weight heparin for the LV thrombus. Coronary CT angiography showed normal epicardial coronary arteries. The next day, CK-MB and troponin I levels normalized to 1.09 ng/mL and 0.267 ng/mL, respectively. Follow-up ECG showed T-wave inversion at the V3-V6 leads in contrast to the normal initial ECG. Sputum studies showed positive results for acid fast stain and polymerase chain reaction for mycobacterium tuberculosis. We started first-line anti-tuberculosis medication (rifampin, isoniazid, ethambutol, and pyrazinamide) immediately. Ten days later, follow-up echocardiography showed normalized LV apical wall motion and no evidence of akinesia of the apex wall (Figure 2C). The left ventricular ejection fraction was 58%. The LV thrombus was still noted on imaging, but decreased in size (Figure 2D). Seventeen days later, we confirmed a diagnosis of pulmonary tuberculosis through positive tuberculosis culture. Drug susceptibility test revealed all sensitive results. He completed a six months of treatment with rifampin, isoniazid, ethambutol and pyrazinamide for two months, followed by rifampin and isoniazid for four months. During the follow-up period, there was no physical evidence of recurrence in TTC.
The Mayo Clinic Criteria are the most widely applied criteria for TTC diagnosis. The proposed diagnostic criteria for TTC include the following: 1) transient hypokinesis, akinesis, or dyskinesis in the LV mid segments with or without apical involvement and regional wall motion abnormality extending beyond a single epicardial vascular distribution; 2) absence of an obstructive coronary lesion or evidence of acute plaque rupture; 3) new electrographic abnormalities and/or elevation in serum cardiac enzymes; and 4) absence of pheochromocytoma or myocarditis3. In our case, the patient met all four criteria for TTC diagnosis: 1) typical balloon-like akinesia of the apex wall; 2) absence of coronary lesion in coronary CT; 3) elevated cardiac enzymes and inverted T-wave in ECG; 4) absence of pheochromocytoma on CT and no evidence of myocarditis.
A common theme among TTC patients is a precipitating stressful event. This event may be either an emotional or a physical stressor, a serious medical or surgical issue, or iatrogenic drug administration2. As a cause of TTC, we could not identify any specific stressful events aside from pulmonary tuberculosis in this patient. Chronic infection such as pulmonary tuberculosis is not generally considered to be a common cause of TTC. However, a few reports suggest that there is a higher plasma norepinephrine concentration in pulmonary tuberculosis patients compared to normal people, which is a typical accepted pathophysiological mechanism of TTC4,5. Thus, we postulate that pulmonary tuberculosis may be a possible precipitant of TTC.
In this patient, cardiac biomarkers were rapidly normalized. In general, the significance of cardiac enzyme elevation is less than in myocardial infarction. The elevation of CK-MB and troponin I are reported around in 53% and 85% of TTC patients1. These findings may suggest that TTC is associated with less myonecrosis and transient elevation of cardiac biomarkers, consequentially. Also, supportive care such as oxygen therapy could improve myocardial ischemia after admission.
Another interesting finding was the development of LV thrombus. There have been some reports of TTC complicated with thrombus6,7,8,9,10,11. The clinical importance of this thrombus is that it may be a potential source of embolic events12. In one study, thromboembolism was found to be the second most frequent cardiovascular complication following TTC12. Despite the fact that left ventricular dysfunction in TTC is a transient phenomenon, it favors the formation of intracardiac mural thrombus through catecholamine-induced platelet activation and low blood flow from wall motion abnormalities in the apical region13. When LV thrombus or significant apical akinesia is present, intravenous heparin followed by oral anticoagulation is recommended until the thrombus and ventricular dysfunction have resolved1.
In conclusion, we report the first case of TTC accompanied by pulmonary tuberculosis, suggesting that pulmonary tuberculosis may be a cause of TTC.
References
1. Bossone E, Savarese G, Ferrara F, Citro R, Mosca S, Musella F, et al. Takotsubo cardiomyopathy: overview. Heart Fail Clin 2013;9:249-266. PMID: 23562126.


2. Tranter MH, Wright PT, Sikkel MB, Lyon AR. Takotsubo cardiomyopathy: the pathophysiology. Heart Fail Clin 2013;9:187-196. PMID: 23562119.


3. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408-417. PMID: 18294473.


4. Miki K, Maekura R, Hiraga T, Hashimoto H, Kitada S, Miki M, et al. Exertional dyspnea-related acidotic and sympathetic responses in patients with sequelae of pulmonary tuberculosis. J Physiol Sci 2010;60:187-193. PMID: 20087706.




5. Watanabe E, Ogawa K, Ban M, Satake T. Sympathetic nervous systems in chronic hypoxic states from pulmonary tuberculosis: a clinical study on plasma norepinephrine and cyclic AMP levels. Jpn J Med 1981;20:180-187. PMID: 6279943.


6. Finsterer J, Stollberger C, Pulgram T. Paraneoplastic takotsubo syndrome with ventricular thrombus and stroke. Herz 2013 11 09 [Epub]. http://dx.doi.org/10.1007/s00059-013-3956-2.


7. Shim IK, Kim BJ, Kim H, Lee JW, Cha TJ, Heo JH. A case of persistent apical ballooning complicated by apical thrombus in takotsubo cardiomyopathy of systemic lupus erythematosus patient. J Cardiovasc Ultrasound 2013;21:137-139. PMID: 24198920.



8. Celik M, Yalcinkaya E, Yuksel UC, Celik T, Iyisoy A. Multiple foci of infarction secondary to giant left ventricular thrombus in a patient with takotsubo cardiomyopathy. Oman Med J 2013;28:294PMID: 23904928.



9. Lee PH, Song JK, Park IK, Sun BJ, Lee SG, Yim JH, et al. Takotsubo cardiomyopathy: a case of persistent apical ballooning complicated by an apical mural thrombus. Korean J Intern Med 2011;26:455-459. PMID: 22205847.




10. Tobar R, Rotzak R, Rozenman Y. Apical thrombus associated with Takotsubo cardiomyopathy in a young woman. Echocardiography 2009;26:575-580. PMID: 19438699.


11. Azzarelli S, Galassi AR, Amico F, Giacoppo M, Argentino V, Giordano G, et al. Apical thrombus in a patient with takotsubo cardiomyopathy. J Cardiovasc Med (Hagerstown) 2008;9:831-833. PMID: 18607250.


12. Mitsuma W, Kodama M, Ito M, Kimura S, Tanaka K, Hoyano M, et al. Thromboembolism in Takotsubo cardiomyopathy. Int J Cardiol 2010;139:98-100. PMID: 18718684.


13. Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest 1996;26:353-370. PMID: 8796362.


Figure┬Ā1
(A) Initial chest radiograph shows numerous nodules in both apices suggesting active pulmonary tuberculosis. (B) Chest computed tomography (CT) shows cavitary lesions in the right upper lobe and multiple small nodules and patchy consolidation in both upper lobes. (C) Chest CT shows an approximately 1.9-cm ovoid filling defect (arrow) in the apex of the left ventricle.
Figure┬Ā2
Transthoracic echocardiography. (A) The typical apical ballooning of Takotsubo cardiomyopathy in the initial apical four-chamber view. (B) The 1.6├Ś1.2-cm thrombus in the apex of the left ventricle is noted in the initial apical two-chamber view. (C) In a follow-up of echocardiography, apical ballooning is completely normalized. (D) On follow-up echocardiography, the thrombus was still noted, but decreased in size. Arrows indicate the apical thrombus.
- TOOLS
-
METRICS

- Related articles
-
Relapases in Pulmonary Tubersulosis
Cavernoplasty for Intractable Cavitary Pulmonary Tuberculosis on 100 Cases1990 December;37(4)
A Case of Caplan`s Syndrome with Pulmonary Tuberculosis1989 September;36(3)
Clinical Study of Lung Cancer Associated with Pulmonary Tuberculosis1989 September;36(3)
Thoracoplasty for Intractable Pulmonary Tuberculosis on 80 Cases1988 December;35(4)


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



