Idiopathic Pleuroparenchymal Fibroelastosis Presenting in Recurrent Pneumothorax: A Case Report
Article information
Abstract
Idiopathic pleuroparenchymal fibroelastosis (PPFE) is a rare, recently classified entity that consists of pleural and subjacent parenchymal fibrosis predominantly in the upper lungs. In an official American Thoracic Society/European Respiratory Society statement in 2013, this disease is introduced as a group of rare idiopathic interstitial pneumonias. We describe a case of a 76-year-old woman with cough and recurrent pneumothorax. She was admitted to our hospital with severe cough at first. High resolution computed tomography (HRCT) disclosed multifocal subpleural consolidations with reticular opacities in both lungs, primarily in the upper lobes, suggesting interstitial pneumonia. Rheumatoid lung was diagnosed initially through an elevated rheumatoid factor, HRCT and surgical biopsy at the right lower lobe. However, one month later, pneumothorax recurred. Surgical biopsy was performed at the right upper lobe at this time. The specimens revealed typical subpleural fibroelastosis. We report this as a first case of idiopathic PPFE in Korea after reviewing the symptoms, imaging and pathologic findings.
Introduction
Idiopathic pleuroparenchymal fibroelastosis (PPFE) is a rare disease characterized by fibrosis involving the pleura and subpleural lung parenchyma due to elastic fiber proliferation. The fibrosis is predominantly located in upper lobes and distributed peripherally1,2.
In 2013 the American Thoracic Surgery/European Respiratory Society classification of idiopathic interstitial pneumonias (IIPs), Idiopathic PPFE is classified as rare IIPs which are introduced newly3. Clinically, patients usually present with chronic respiratory symptoms such as shortness of breath and dry cough. Also recurrent respiratory infection and spontaneous pneumothorax are common. Some patients have family history of interstitial lung disease and nonspecific autoantibodies4.
The diagnosis of this disease is established on the basis of compatible radiologic and histopathologic findings. High resolution computed tomography (HRCT) shows subpleural consolidations with parenchymal distorsion usually in upper lobes. Histological characteristics are homogenous subpleural fibrosis and dense masses of elastic fibers on elastic stain3.
This disease is unusual and not widespread yet, so it is likely to be misrecognized as other common interstitial lung diseases. We here describe a case of this rare disease that is misdiagnosed initially and confirmed as idiopathic PPFE through surgical lung biopsy at the right upper lobe.
Case Report
A 76-year-old female patient was admitted with a 3-year history of nonproductive coughing. She had no other symptoms such as sputum, dyspnea or fever. She never smoked and was not taking any medicine. Physical examination showed clear breathing sound on both lungs. Routine biochemistry and hematology profile was normal. Anti nuclear antibody was positive and a titer was 1:80 in a speckled pattern. Rheumatoid factor was 30.4 IU/mL (normal range, 0.1-15 IU/mL). Other autoimmune markers were all negative.
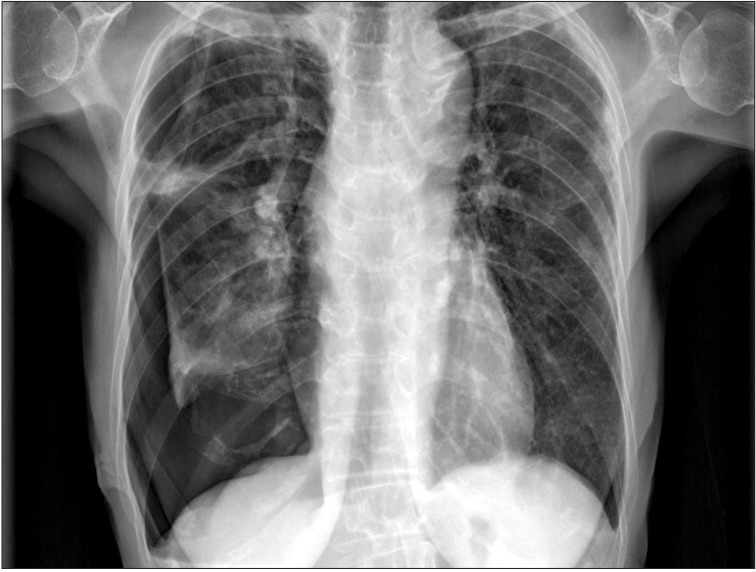
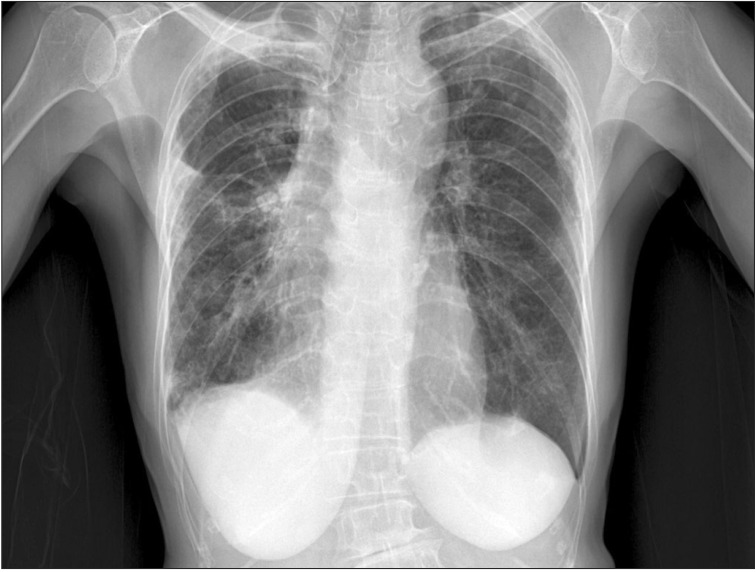
Her chest X-ray showed multifocal consolidations and reticulonodular infiltrations in peripheral portion of both lungs (Figure 1). Chest HRCT also showed multifocal subpleural consolidations with reticular opacities in both lungs, predominantly in upper lungs. Architectural distorsion and traction bronchiectasis were combined. There was no definite honeycomb appearance. Small localized pneumothorax in right lung was also observed (Figure 2). Patient underwent bronchoalveolar lavage which showed 36% lymphocytes, 1% neutrophils, 62% macrophages, and 1% eosinophils. The CD4/CD8 ratio was 0.71. Video-assisted thoracoscopic surgery was performed at the right lower lobe. The specimen revealed dense fibrosis in the subpleura, lung parenchyme and interstitium with temporal heterogeneity (Figure 3). She was treated with prednisolone and azathioprine under the impression of rheumatoid lung, on the basis of elevated rheumatoid factor, HRCT and the biopsy results. Coughing was improved slightly after treatment. After 1 month, she complained of dyspnea and pleuritic chest pain. Physical examination revealed decreased breathing sound on right lung. Chest X-ray showed a large size of pneumothorax in right lung (Figure 4). Chest tube was inserted for re-expansion. She underwent video-assisted thoracoscopic wedge resection of right upper lobe in this time for exact diagnosis. Dense pleural adhesions at apex and inflammatory changes at visceral pleura were observed grossly during operation. The biopsy revealed marked subpleural thickening and homogenous fibrosis. Dense elastic fibers were seen in the subpleura and adjacent parenchyma on elastic stain (Figure 5).
Chest radiograph shows multifocal consolidations and reticulonodular infiltrations in peripheral portion of both lungs. These lesions show upper lobe predominancy. Small pneumothorax is noted in right lung.
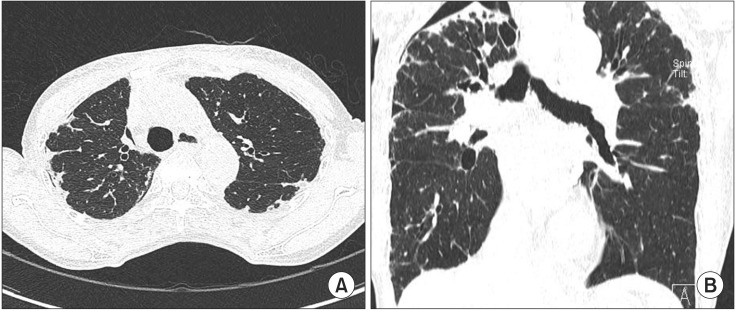
(A) High resolution computed tomography (HRCT) shows multifocal subpleural consolidations with reticular opacities in both lungs and small pneumothorax in right lung. Architectural distortions and traction bronchiectasis are combined in both lungs. (B) Coronal HRCT shows that these lesions predominantly affect the upper lobes.
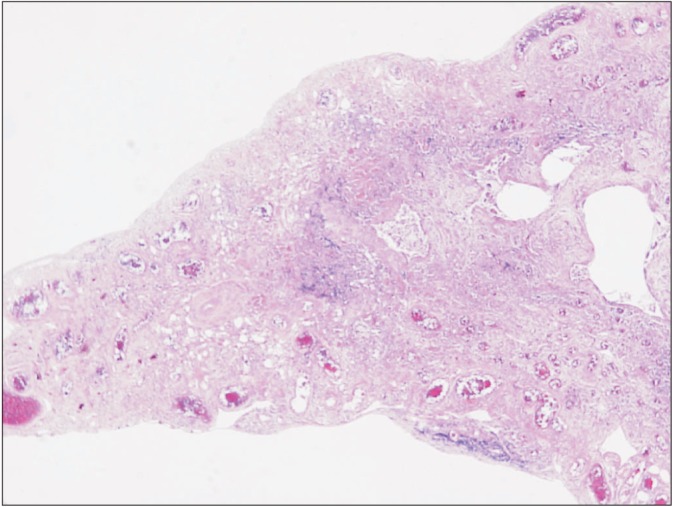
Surgical lung biopsy specimen (H&E stain, ×40) at low power demonstrated dense fibrosis in the subpleura, lung parenchyme, and interstitium with temporal heterogeneity.
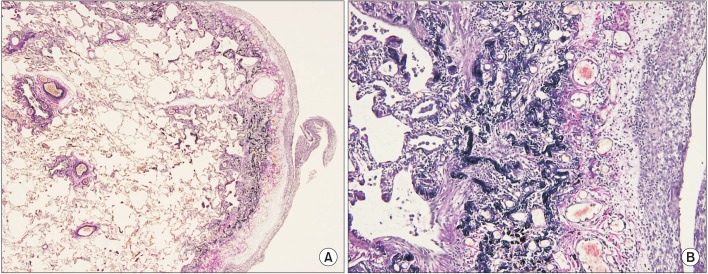
Surgical lung biopsy specimen (Elastic Van Gieson stain) at low power (A, ×100) shows pleural thickening and subpleural fibrosis. High power (B, ×200) demonstrated dense masses of elastic fibers beneath the thickend pleura.
In summary, she had chronic dry cough and recurrent pneumothorax. Multiple subpleural consolidations were seen predominantly in upper lobes on imaging study. Also the specimens obtained from the right upper lobe by surgical lung biopsy revealed dense fibrosis and elastic fibers in subpleura on elastic stain with normal lung parenchyma. Idiopathic PPFE was diagnosed based on clinical manifestation, HRCT and biopsy results. We report here a first case of idiopathic PPFE in Korea.
Discussion
Idiopathic PPFE is a rare IIP that was classified recently, but it is not a entirely new lung disease. PPFE was mentioned in Japanese literature by Amitani et al. as idiopathic upper lobe fibrosis in 19924. Idiopathic PPFE was first described in the English medical literature by Frankel et al.1 in 2004. They reported five patients with unique radiologic and histopathologic findings and defined Idiopathic PPFE as a distinct entity1. Another small cases were published in 2008, followed by a report of 2 cases in 20112,5. More recently, a study was performed to review several cases which meet published criteria for PPFE in London Brompton Hospital4. However, this unusual disease was not reported in Korea yet.
Common symptoms of idiopathic PPFE are dyspnea on exertion and chronic cough. During the course of the disease, spontaneous pneumothorax and recurrent respiratory infections occur often. Pneumothorax is rarely seen in other IIPs, so it is distinguishing feature of PPFE. The important things to make a diagnosis are imaging and surgical lung biopsy. Marked bilateral apical pleural thickening associated with fibrosis is key radiologic finding. Also architectural distorsion, traction bronchiectasis, reticular opacities and honeycombing may be seen. These features are similar to those of other idiopathic pulmonary fibrosis. But in PPFE, these characteristics present mainly in upper lobes with the involvement of the lower lobes being absent or less marked1,5. The differentiating pathologic features include thickened pleura by a mixture of elastic and dense collagen fibers. The entire pleura appears as a homogeneously thick elastic band. This is key diagnostic finding of idiopathic PPFE. The adjacent lung parenchyma is spared and the border between fibroelastosis and normal parenchyma is abrupt6.
The radiographic appearance in our case showed multifocal subpleural consolidations with reticular opacities predominantly in both upper lungs with architectural distorsion and traction bronchiectasis. This finding was initially considered as nonspecific interstitial pneumonia (NSIP) or usual interstitial pneumonia (UIP). Clinically, we thought about high probability of NSIP associated with rheumatoid arthritis because of lymphocyte dominant bronchoalveloar lavage fluid and elevated rheumatoid factor. However, the surgical lung biopsy showed subpleural dense fibrosis and lymphocyte infiltration, suggesting UIP. Thus, the immunosuppressive treatment started for rheumatoid arthritis related interstitial lung disease.
The elastic stain did not performed in this time because we did not recognize about PPFE at first. But spontaneous pneumothorax recurred at right lung and upper lobe predominant subpleural fibrosis implied a possibility of PPFE, so second surgical biopsy at the right upper lobe and elastic stain were done. Finally, homogenous subpleural fibroelastosis which is typical finding of PPFE was identified.
Although UIP show also a subpleural-predominant interstitial fibrosis, it is temporally heterogeneous in its fibrosing pattern1. And UIP primarily affects the lower lobes and leads to extensive remodelling of the lung parenchyma, eventually resulting in end-stage fibrosis with effacement of the original parenchymal architecture. These features are not characteristics of PPFE. PPFE has a typical upper zone predominance and sparing of the parenchyma distant from the pleura.
Similar to PPFE, NSIP can have a subpleural distribution fibrosis but it is temporally homogeneous and lacks the fibroelastotic component1. It consist of collagen fibers, not elastic fibers. Lymphocytic infiltration indicating inflammation can be seen in both, but it is not specific findings. Also NSIP typically has bilateral lung involvement with a lower-lobe predilection and pneumothorax is rarely occurred. However, according to literature by Reddy et al.4 some patients with PPFE could have coexistent interstitial lung disease and nonspecific auto-antibodies.
The etiology of this disorder remains poorly understood, but not all cases of PPFE are idiopathic.
Previously reported data suggest that recurrent infections, genetic predisposition and autoimmune mechanism may have a role in pathogenesis4. In addition, chemotherapeutic drugs, external beam radiation and a chronic graft-versus-host disease also can cause pleural fibrosis6.
Our patient showed positivity for only rheumatoid factor among autoantibodies and had no symptoms such as arthritis. Furthermore she did not have family history of lung disease, past history of chemotherapy or radiation therapy. There is no evidence of infection. Hence we diagnosed her as a idiopathic PPFE.
Idiopathic PPFE is lately recognized as a distinct entity and very rare, thus it is easy to be underdiagnosed or misdiagnosed. The possibility of idiopathic PPFE should be considered when pulmonary fibrosis is predominant in upper lobes in addition to known UIP or NSIP pattern. Also accurate surgical biopsy at the affected area is recommended. Performing elastic stain is very useful for establishing the diagnosis. Clinicians should be aware of this disease and differentiating it from other interstitial lung disease.
Notes
No potential conflict of interest relevant to this article was reported.