 |
 |
| Tuberc Respir Dis > Volume 77(5); 2014 > Article |
|
Abstract
Aspergillus tracheobronchitis is a form of invasive pulmonary aspergillosis in which the Aspergillus infection is limited predominantly to the tracheobronchial tree. It occurs primarily in severely immunocompromised patients such as lung transplant recipients. Here, we report a case of Aspergillus tracheobronchitis in a 42-year-old man with diabetes mellitus, who presented with intractable cough, lack of expectoration of sputum, and chest discomfort. The patient did not respond to conventional treatment with antibiotics and antitussive agents, and he underwent bronchoscopy that showed multiple, discrete, gelatinous whitish plaques mainly involving the trachea and the left bronchus. On the basis of the bronchoscopic and microbiologic findings, we made the diagnosis of Aspergillus tracheobronchitis and initiated antifungal therapy. He showed gradual improvement in his symptoms and continued taking oral itraconazole for 6 months. Physicians should consider Aspergillus tracheobronchitis as a probable diagnosis in immunocompromised patients presenting with atypical respiratory symptoms and should try to establish a prompt diagnosis.
Aspergillus tracheobronchitis is a rare clinical form of invasive aspergillosis in which the Aspergillus infection is limited mainly to the tracheobronchial tree1. It occurs primarily in immunocompromised hosts such as lung transplant recipients and patients afflicted with acquired immunodeficiency syndrome, hematologic and non-hematologic malignancy, and chronic obstructive airway disease1. However, it can also occur in mildly immunocompromised hosts such as diabetic patients and in immunocompetent hosts. Here, we report a case of Aspergillus tracheobronchitis in a mildly immunocompromised patient with diabetes, who presented with intractable cough, unexpectorated sputum, and chest discomfort and was successfully treated with oral itraconazole.
A 42-year-old man presented with a severe cough over a period of 2 months. His past medical history included pulmonary tuberculosis for which treatment was completed 1 year prior and diabetes mellitus, which was treated with insulin for over 14 years.
On admission, he had a severe cough, unexpectorated sputum, and chest discomfort. All his vital signs were stable except for a low-grade fever. He had a very thin physique and a chronic ill-looking appearance. Chest auscultation revealed fine crackle sounds in the left lung fields.
Laboratory work-up revealed the following: leukocyte count, 12,160 cells/mm3 (neutrophils 75%, lymphocytes 16.5%, monocytes 5.7%, eosinophils 2.5%, and basophils 0.2%); hemoglobin level, 12.3 g/dL; glycosylated hemoglobin, 10.1%; platelet count, 422,000 cells/µL; C-reactive protein level, 3.95 mg/dL; and pro-calcitonin quantitative level, <0.05. The other blood chemistry values were within normal limits, and the human immunodeficiency virus test and serum galactomannan index were negative. Three sputum acid-fast bacilli (AFB) tests were also negative.
A chest radiograph revealed a destroyed tuberculosis scar in the left and right middle lung fields, and this finding showed no interval change compared to that in a chest radiograph obtained a year ago (Figure 1A, B). A chest computed tomography scan showed a fibrotic cavity, traction bronchiectasis, and multiple small nodules with destructive changes in both lungs (Figure 1C).
Empirical antibiotic therapy was initiated immediately on suspicion of community-acquired pneumonia; the patient did not show any improvement with this treatment.
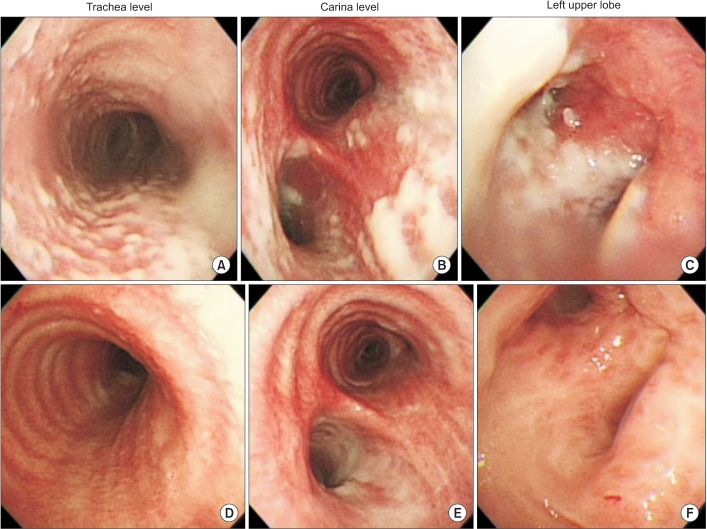
On the seventh hospital day, the patient underwent a flexible bronchoscopy for an evaluation of causality. The bronchoscopic examination demonstrated multiple, discrete, gelatinous whitish plaques involving mainly the trachea and left bronchus (Figure 2A-C). We performed a biopsy of the tracheal lesions, which revealed only chronic active inflammation with necrotic tissues. The AFB, periodic acid-Schiff, and Grocott's methenamine silver stains of the biopsy tissue were all negative.
The patient's symptoms did not improve in spite of empirical antibiotic treatment. On the 14th hospital day, he underwent a second bronchoscopic examination. There was no interval change compared to previous bronchoscopic findings. Another biopsy performed at this time revealed the same findings as the previous biopsy. Although the pathology was not confirmed, we made a presumptive diagnosis of Aspergillus tracheobronchitis on the basis of his symptoms, discriminative bronchoscopic findings, and unresponsiveness to antibiotics. On the 16th hospital day, empirical antifungal therapy with intravenous amphotericin B was initiated, and antibiotic administration was discontinued. However, 5 days later, we changed the drug to itraconazole owing to drug fever and renal insufficiency related to amphotericin B administration. After 7 days of intravenous itraconazole administration, the intractable cough, unexpectorated sputum, and chest discomfort symptoms started showing improvement. On the 35th hospital day, he underwent a follow-up bronchoscopic examination, which revealed a significant improvement in the numerous gelatinous whitish plaques in the trachea and left bronchus (Figure 2D-F). On the 44th hospital day, the patient was discharged on oral itraconazole with significant improvement of his symptoms. After 2 months, the Aspergillus species was isolated upon microbiological assessments of the sputum, washing fluid, and biopsy specimens. We finally confirmed the diagnosis of Aspergillus tracheobronchitis and continued treatment with oral itraconazole for 6 months. Oral itraconazole was discontinued after the patient demonstrated clinical improvement, bronchoscopic resolution, and confirmation of microbiologic eradication.
Aspergillus tracheobronchitis is a rare form of invasive pulmonary aspergillosis in which the Aspergillus infection is limited entirely or predominantly to the tracheobronchial tree. Wheaton2 first reported Aspergillus tracheobronchitis in a 2.5-year-old girl who died of pneumonia in 1890. The incidence of Aspergillus tracheobronchitis occurs in less than 7% of pulmonary aspergillosis cases1. It usually occurs in patients with hematological malignancy with neutropenia, bone marrow or solid organ transplant recipients, and in those receiving corticosteroid therapy3. However, it can occur in lesser immunocompromised patients such as those with post-influenza symptoms, chronic obstructive pulmonary disease, and diabetes and in the elderly4,5,6,7.
In this case, we think that Aspergillus tracheobronchitis was caused by uncontrolled diabetes and a severely destroyed scar of tuberculosis suitable for Aspergillus colonization.
The clinical manifestations of this infection are usually nonspecific, and includes cough, exertional dyspnea, white or purulent sputum, fever, wheezing, and night sweats. Our patient also had the same clinical manifestations such as intractable cough, unexpectorated sputum, and chest discomfort.
Wu et al.8 has proposed a classification of the intraluminal lesions, based on bronchoscopic morphology, into four types: the superficial infiltration type, full-layer involvement type, occlusion type, and mixed type. Our case can be classified as the superficial infiltration type.
Aspergillus tracheobronchitis is diagnosed by one of the following: histologic evidence of tissue invasion by Aspergillus on a biopsy, histology suggestive of aspergillosis associated with positive cultures of the Aspergillus species, clinical and radiological findings strongly suggestive of invasive aspergillosis associated with microbiologic identification in bronchoalveolar lavage, or a positive galactomannan serum assay9,10. In our case, we did not obtain pathologic confirmation of the Aspergillus species. However, the Aspergillus species was isolated upon microbiological assessments of the sputum, washing fluid, and biopsy specimens. Tasci et al.11 reported that microscopic examination of respiratory specimens is also a useful sensitive tool to confirm the diagnosis. In addition, the patient's bronchoscopic findings improved after antifungal therapy. Moreover, he did not respond to conventional antibiotics. We think that Aspergillus hyphae were not visualized on the biopsy specimens because of inappropriate circumference for proliferation owing to his relatively mild immunocompromised state.
The outcome of antifungal therapy depends largely on the patient's immune status. Amphotericin B was the treatment of choice for invasive aspergillosis in the past, but recently, voriconazole has been recommended as the primary treatment regimen9.
However, we were unable to use voriconazole owing to health insurance coverage restrictions. We first initiated antifungal therapy with IV amphotericin B. However, we changed IV amphotericin B to itraconazole owing to the development of drug fever and acute renal insufficiency after 5 days. Kramer et al.12 reported that oral therapy with itraconazole, administered for 6-12 months, was effective in invasive aspergillosis after lung transplantation. However, the optimal duration of therapy has not been defined. Most experts attempt to treat until resolution or stabilization of all clinical and radiographic manifestations13. Aspergillus tracheobronchitis is considered a rare disease these days. However, we can predict that the incidence of Aspergillus tracheobronchitis may rise owing to the increasing number of immunocompromised hosts such as transplant recipients, and cancer, human immunodeficiency virus-infected, and elderly patients. Physicians should therefore consider Aspergillus tracheobronchitis as a possible diagnosis in immunocompromised patients presenting with atypical respiratory symptoms and should attempt a prompt diagnosis with a procedure such as bronchoscopy.
References
1. Kemper CA, Hostetler JS, Follansbee SE, Ruane P, Covington D, Leong SS, et al. Ulcerative and plaque-like tracheobronchitis due to infection with Aspergillus in patients with AIDS. Clin Infect Dis 1993;17:344-352. PMID: 8218674.



2. Wheaton SW. Case primarily of tubercle, in which a fungus (Aspergillus) grew in the bronchi and lung, simulating actinomycosis. Trans Pathol Soc Lond 1890;41:34-37.
3. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002;121:1988-1999. PMID: 12065367.


4. Boots RJ, Paterson DL, Allworth AM, Faoagali JL. Successful treatment of post-influenza pseudomembranous necrotising bronchial aspergillosis with liposomal amphotericin, inhaled amphotericin B, gamma interferon and GM-CSF. Thorax 1999;54:1047-1049. PMID: 10525567.



5. Franco J, Munoz C, Vila B, Marin J. Pseudomembranous invasive tracheobronchial aspergillosis. Thorax 2004;59:452PMID: 15115885.



6. Lee SE, Jun EJ, Song JH, Shin JW, Kim JY, Park IW, et al. A case of pseudomembranous necrotizing bronchial aspergillosis in an old age host. Tuberc Respir Dis 2007;63:278-282.

7. Chang SM, Kuo HT, Lin FJ, Tzen CY, Sheu CY. Pseudomembranous tracheobronchitis caused by Aspergillus in immunocompromised patients. Scand J Infect Dis 2005;37:937-942. PMID: 16308239.


8. Wu N, Huang Y, Li Q, Bai C, Huang HD, Yao XP. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect 2010;16:689-695. PMID: 19689467.


9. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011;20:156-174. PMID: 21881144.



10. Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med 2006;173:707-717. PMID: 16387806.


11. Tasci S, Glasmacher A, Lentini S, Tschubel K, Ewig S, Molitor E, et al. Pseudomembranous and obstructive Aspergillus tracheobronchitis: optimal diagnostic strategy and outcome. Mycoses 2006;49:37-42. PMID: 16367817.

12. Kramer MR, Denning DW, Marshall SE, Ross DJ, Berry G, Lewiston NJ, et al. Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am Rev Respir Dis 1991;144(3 Pt 1):552-556. PMID: 1654038.


13. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327-360. PMID: 18177225.



Figure 1
(A) Chest radiograph obtained 1 year ago showing a destroyed tuberculosis scar in the left lung and right middle and lower lung fields. (B) Chest radiograph obtained upon admission does not show any significant changes from the radiograph obtained in the previous year. (C) Chest computed tomography image showing a fibrotic cavity, traction bronchiectasis, and multiple small nodules with destructive changes in both lungs.

Figure 2
(A-C) Bronchoscopic examination images obtained upon admission showing multiple, discrete, gelatinous, whitish plaques mainly involving the trachea and the left bronchus. (D-F) Follow-up bronchoscopic examination images showing significant improvement compared to numerous gelatinous whitish plaques in the trachea and the left bronchus that were seen earlier.
- TOOLS
-
METRICS

- Related articles
-
Pneumonia in the Immunocompromised Host.1996 December;43(6)
Herpes Simplex Virus Pneumonia in Immunocopmromised Host.1999 January;46(1)
A Case of aspergillus tracheobronchitis in non-immunocompromise patient.2000 October;49(4)
Pseudomembranous Aspergillus Tracheobronchitis in an Immunocompetent Patient.2008 November;65(5)
Disseminated
Mycobacterium intracellulare Infection in an Immunocompetent Host2012 May;72(5)


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



