 |
 |
| Tuberc Respir Dis > Volume 78(2); 2015 > Article |
|
Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitive disease showing various radiographic and clinical manifestations. Its clinical course has not been fully understood. Here I describe a case of a 23-year-old immunocompetent man with frequently relapsing ABPA. He was asthmatic. He visited our hospital because of a chronic cough. Laboratory examination showed eosinophilia with increased total and Aspergillus-specific IgE as well as positive skin reaction to Aspergillus fumigatus. Radiologic feature was a dense consolidation. Histology showed organizing pneumonia with eosinophilic infiltration. On the diagnosis of ABPA, he was treated with systemic steroid and itraconazole. Although treatment response was excellent, he suffered from recurrent ABPA three times thereafter in the form of fleeting mass-like consolidation.
Allergic bronchopulmonary aspergillosis (ABPA) is hypersensitivity disorder against the antigens of Aspergillus fumigatus complicating the course of various pulmonary disorders including bronchial asthma and cystic fibrosis. The pathogenesis of ABPA is composed of both the compromised host defense, which causes the colonization of Aspergillus in the bronchial tree, and the allergic host reaction to Aspergillus antigen1. The pulmonary infiltrates seen in ABPA usually clear within 1 to 2 months after a moderate dose of corticosteroid2. However, clinical features are highly variable and not easy to predict the relapse. Even after successful treatment, ABPA may relapse in 20%-40% of patients and can be recurred in several years after remission3,4. Although previous studies suggested that bronchiectasis and high attenuation mucus may be related to frequent relapse, it has not been clarified the radiologic patterns which are helpful to predict the high probability of relapse.
I report the case of a 23-year-old, immunocompetent man with ABPA presenting recurrent episode four times in the form of mass-like consolidation for 4 years. I discuss my case with the previous literatures focusing on the clinical and radiological features.
A 23-year-old man was referred to our hospital because of chronic cough for 6 months and markedly elevated eosinophil count. Sixteen years ago, he had been diagnosed with asthma and had used salbutamol intermittently. He was a lifelong non-smoker.
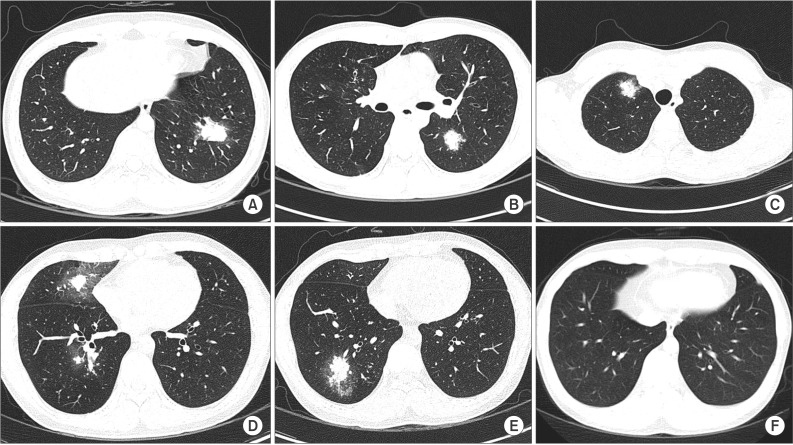
On examination, the white blood-cell count was 7,610/┬ĄL with a 37.1% of eosinophil (2,410/┬ĄL). Screening test results for parasites were negative. Total IgE was 2,500 IU/mL and the serum level of Aspergillus-specific IgE was elevated (>50 kU/L). Aspergillus-specific IgG was also elevated (>200 mg/dL). Allergy skin prick test showed positive reactions to A. fumigatus. Pulmonary function test showed obstructive patterns with positive bronchodilator response, i.e., 410 mL (20%) increase from 2.00 L to 2.41 L of forced expiratory volume in 1 second (FEV1) of FEV1 after inhalation of beta2-agonist, 0.72 of FEV1/forced vital capacity ratio, decreased forced expiratory flow between 25% and 75% of functional vital capacity (FEF25-75%, 1.70 L; 36% of predictive value), and positive mannitol bronchial provocation test. High resolution computed tomography (HRCT) showed 3.3├Ś2.0-cm-sized, ill-defined consolidation and peripheral ground glass opacity in the left lower lobe lateral basal segment (Figure 1A). Central or peripheral bronchiectasis was not demonstrated. The patient was referred for a video-assisted thoracoscopic biopsy of the consolidation in the left lower lobe. Histologic features were organizing pneumonia filled with eosinophil-dominant inflammatory exudates. His clinical and laboratory findings fulfilled the six major diagnostic criteria of ABPA proposed by Rosenberg-Patterson5 (i.e., asthma, pulmonary infiltration, immediate cutaneous hyperreactivity to Aspergillus, peripheral eosinophilia, elevated serum IgE over 1,000 IU/mL, serum A. fumigatus-specific IgG and IgE). On the diagnosis of ABPA, he began to be treated with 400 mg/day itraconazole and 1 mg/kg/day oral methylprednisolone. After 3 months later, consolidation in left lower lobe was completely disappeared. Blood eosinophil count became normalized (0.7%, 90/┬ĄL) and serum IgE level was decreased (1,632 IU/mL). So, steroid was slowly tapered and eventually wean. Regular radiologic and serologic work-up was performed thereafter.
After 1 year after completion of treatment, HRCT demonstrated 2.0├Ś1.8-cm-sized ill-defined consolidation containing peripheral ground glass opacity in left lower lobe superior segment (Figure 1B). Laboratory test showed a peripheral eosinophilia (19.2%, 1,180/┬ĄL), high total IgE level (>3,000 IU/mL). After considering the radiology and serology, a recurrence of ABPA was diagnosed. After re-treated with itraconazole and oral methylprednisolone in 1 month, HRCT showed remarkable improvement. He was treated with oral methylprednisolone at a maintenance dose for 6 months and tapered.
After 8 month after second treatment, 2.1├Ś1.5-cm-sized, ill-defined consolidation in the right upper lobe apical segment and small nodules and peripheral ground glass opacity in right upper lobe posterior segment was shown (Figure 1C). Serologic evaluation showed eosinophilia (13.9%, 1,240/┬ĄL) and elevated total IgE (>3,000 IU/mL). As a result, third attack of ABPA was diagnosed. After retreatment with oral methylprednisolone and itraconazole, consolidation was disappeared.
After 3 months after completion of third treatment, consolidations were developed in right middle lobe and right lower lobe (Figure 1D, E). Eosinophilia (25.1%, 1,850/┬ĄL) and elevated IgE (>3,000 IU/mL) was shown again. He was re-treated with oral methylprednisolone and itraconazole. After 3 months later, follow-up radiologic evaluation demonstrated complete remission. The bronchiectasis was not developed (Figure 1F).
The most interesting feature of our case is that mass-like consolidation appeared repeatedly with various sizes and at different locations during exacerbation period even after several months of remission. The common findings of ABPA on HRCT of the chest include central bronchitectasis with peripheral tapering of bronchi, mucoid impaction, presence of centrilobular nodules, and tree-in-bud opacities. Mass or consolidation is uncommonly found2,3. Fleeting pulmonary infiltrate in upper or middle lobe is known as the characteristic feature of the disease during acute exacerbation. In our patient, it is peculiar that radiologic feature in recurrent phase was manifested as frequently relapsed fleeting masses after short duration of remission although the response to steroid is excellent. It is not certain whether radiographic patterns at diagnosis reflect the severity of innate immune response and is helpful to predicting progression or recurrence. Although radiographic features of recurrent ABPA have not been well characterized, there are some evidences that computed tomography findings at diagnosis can be a clue to predict frequent relapses in ABPA. Bronchiectasis and high attenuation mucus in ABPA is known to be associated with not only serologic severity but also frequent relapse6. In addition, another study showed that a chest radiographic severity staging system based on severity and duration of changes may be useful in assess the activity of the disease and help to predict the likelihood of progression of the disease7. However, the clinical manifestation of the ABPA forming dense consolidation is not well characterized yet. Our case suggests that mass-like consolidation, in addition to known risk factors such as bronchiectasis or high attenuation mucus, may be one of the important features having a tendency of frequent relapse.
The complexity of clinical and radiologic feature of ABPA may be originated from complex immunological reaction to chronic airway colonization by Aspergillus species2,8. The genetic predispositions also have a role in presenting different radiological severity and frequency of relapse. Cystic fibrosis transmembrane conductance regulator (CFTR) mutation affecting mucus quality is more prevalent in ABPA patients9. High prevalence of polymorphism of interleukin (IL)-4RA, IL-10, surfactant protein A2, and toll-like receptor in ABPA patients is thought to be responsible for the exaggerated interaction with A. fumigatus, altered immune response and compromise host defense10,11,12. Thus, radiologic presentation may a result of reproduced inflammatory process between Aspergillus species and bronchial epithelium that are influenced by genetic predisposition.
Systemic corticosteroid is a mainstay of therapy, which not only attenuate of the immunological activity in ABPA but also suppress the inflammation. Most patient in stage I and III ABPA can go complete remission which is defined as clearing of pulmonary radiographic alterations and respiratory symptoms at least 6 months with a 35% to 50% decline in IgE level by 6 weeks5. Our patient was also responded well in systemic corticosteroid and antifungal agent and maintained complete remission in several months. However, serum IgE level did not significantly decreased but maintained the elevated level above 1,000 IU/mL (1,228-1,632 IU/mL). Several studies demonstrated that the immunologic situation do not return to fully inactivated state after remission. Titers of IgA against A. fumigatus in bronchoalveolar fluid, number of granulocyte in bronchoalveolar fluid and IgE antibodies in the serum is still elevated during remission stage13. Even in serologic ABPA which have been regarded as less aggressive form, recent study showed that almost 39% of ABPA-S patients experienced relapse4. Our case give a supporting evidence that close observation and long-term follow-up is mandatory in consolidative ABPA even if the initial response to steroid is satisfactory enough to normalize the radiologic abnormalities.
Bronchiectasis was not demonstrated in our patients even after 4 times flair-up events occurring dense eosinophilic consolidations. It may be due to close monitoring and early treatment of ABPA. Inadequate treatment of ABPA is known to relate to development of bronchiectasis in areas of pulmonary infiltrates on long-term follow-up. Conversely, there are some evidences that early treatment with oral glucocorticoid may prevent to development of central bronchiectasis4,6. The mechanistic roles in damaging bronchial walls, causing bronchiectasis, causing bronchiolitis obliterans, and supporting growth of A. fumigatus are not understood sufficiently. Host immune responses and direct fungal injury is known to related to airway injury and fibrosis in ABPA. Disruption of bronchial epithelium in ABPA may be believed to be results of a monokine inflammatory response and Th2 CD4+ T lymphocyte-medicated inflammation. Aspergillus proteins induce interaction with respiratory epithelium and disruption of epithelial integrity resulting in the influx of exudate such as serum proteins and extracellular matrix protein to the lumen during exacerbation. Because inflammatory process of airway epithelium is able to promote the immune interaction between A. fumigatus and damaged airway mucosa, patients with ABPA can suffer from long-lasting periods of exacerbation if untreated14.
In summary, this is the uncommon case showing unique clinical patterns. I think that consideration of this pattern is helpful for diagnosis and management of ABPA presenting as mass-like consolidation.
References
1. Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2002;110:685-692. PMID: 12417875.


2. Bains SN, Judson MA. Allergic bronchopulmonary aspergillosis. Clin Chest Med 2012;33:265-281. PMID: 22640845.


3. Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest 2006;130:442-448. PMID: 16899843.


4. Agarwal R, Garg M, Aggarwal AN, Saikia B, Gupta D, Chakrabarti A. Serologic allergic bronchopulmonary aspergillosis (ABPA-S): long-term outcomes. Respir Med 2012;106:942-947. PMID: 22445696.


6. Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, Jindal SK. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest 2007;132:1183-1190. PMID: 17646221.


7. Kiely JL, Spense L, Henry M, Hurley MF, Kelleher N, Bredin CP. Chest radiographic staging in allergic bronchopulmonary aspergillosis: relationship with immunological findings. Eur Respir J 1998;12:453-456. PMID: 9727800.


8. Patterson R, Greenberger PA, Lee TM, Liotta JL, OŌĆÖNeill EA, Roberts M, et al. Prolonged evaluation of patients with corticosteroid-dependent asthma stage of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 1987;80:663-668. PMID: 3316345.


9. Miller PW, Hamosh A, Macek M Jr, Greenberger PA, MacLean J, Walden SM, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in allergic bronchopulmonary aspergillosis. Am J Hum Genet 1996;59:45-51. PMID: 8659542.


10. Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol 1999;104:1008-1014. PMID: 10550746.


11. Saxena S, Madan T, Shah A, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of SP-A2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2003;111:1001-1007. PMID: 12743564.


12. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 2008;197:618-621. PMID: 18275280.



13. Kauffman HF, Beaumont F, de Monchy JG, Sluiter HJ, de Vries K. Immunologic studies in bronchoalveolar fluid in a patient with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 1984;74:835-840. PMID: 6334111.


14. Persson CG, Erjefalt JS, Erjefalt I, Korsgren MC, Nilsson MC, Sundler F. Epithelial shedding: restitution as a causative process in airway inflammation. Clin Exp Allergy 1996;26:746-755. PMID: 8842547.


- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 5,135 View
- 22 Download
- Related articles
-
Diagnosis and Treatment of Allergic Bronchopulmonary Aspergillosis.1998 August;45(4)
Allergic Bronchopulmonary Aspergillosis Associated with Aspergilloma.2004 March;56(3)


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



