 |
 |
| Tuberc Respir Dis > Volume 80(1); 2017 > Article |
|
Abstract
Background
Inhaled indacaterol (Onbrez Breezhaler), a long-acting β2-agonist, is approved in over 100 countries, including South Korea, as a once-daily bronchodilator for maintenance and treatment of chronic obstructive pulmonary disease (COPD). Here, we present an interim analysis of a post-marketing surveillance study conducted to evaluate the real-world safety and effectiveness of indacaterol in the Korean population.
Methods
This was an open-label, observational, prospective study in which COPD patients, who were newly prescribed with indacaterol (150 or 300 µg), were evaluated for 12 or 24 weeks. Safety was assessed based on the incidence rates of adverse events (AEs) and serious adverse events (SAEs). Effectiveness was evaluated based on physician's assessment by considering changes in symptoms and lung function, if the values of forced expiratory volume in 1 second were available.
Results
Safety data were analyzed in 1,016 patients of the 1,043 enrolled COPD patients receiving indacaterol, and 784 patients were included for the effectiveness analysis. AEs were reported in 228 (22.44%) patients, while 98 (9.65%) patients reported SAEs. The COPD condition improved in 348 patients (44.4%), while the condition was maintained in 396 patients (50.5%), and only 40 patients (5.1%) exhibited worsening of ailment as compared with baseline. During the treatment period, 90 patients were hospitalized while nine patients died. All deaths were assessed to be not related to the study drug by the investigator.
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterized by persistent airflow limitation which is associated with an enhanced chronic inflammatory response to noxious particles or gases1. According to the Global Burden of Disease Study reports, COPD is projected to become the third leading cause of mortality worldwide by 20201. In South Korea, the prevalence of COPD has been reported as 12.2%-15.5% in 2012, as per the 5th Korean National Health and Nutrition Examination Survey (KNHNES)2. Bronchodilators remain the cornerstone of pharmacological management of COPD patients. The current Global Initiative for Chronic Obstructive lung Disease (GOLD) guidelines recommend inhaled long-acting bronchodilators such as long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) for management of patients with moderate-to-severe COPD1. Once-daily indacaterol is the first approved LABA, inhaled using the Breezhaler device (Onbrez Breezhaler; Novartis, Basel, Switzerland) for maintenance bronchodilator treatment of COPD patients3. It was first approved in the European Union in 2009 as a once-daily treatment at doses of 150 and 300 µg4; since then, it has been approved in over 100 countries worldwide for the maintenance treatment of COPD5. Indacaterol received approval for maintenance bronchodilator treatment of COPD patients in South Korea in 2010.
In clinical studies, once-daily indacaterol showed statistically significant and clinically meaningful improvements in lung function, improved dyspnoea, reduced rescue medication use and improved quality of life (QoL) in patients with moderateto-severe COPD4,6. In a 12-week study conducted in Asian patients with moderate-to-severe COPD, including patients from Korea, indacaterol (150 and 300 µg) showed effective bronchodilation and improvements in patient-reported outcomes. Moreover, indacaterol had a good safety profile and tolerability in this population7. Furthermore, in a 12-week post-hoc analysis conducted in an Asian COPD population, indacaterol treatment demonstrated clinically important improvements in lung function, dyspnoea, and health status8.
This post-marketing surveillance (PMS) was conducted to meet the local Health Authority regulatory requirements in South Korea, in order to identify unexpected adverse events (AE), if any, and their frequency as well as to understand the factors that may influence the safety and effectiveness of indacaterol in COPD patients. The report presented here is an interim analysis of this PMS conducted in COPD patients in South Korea.
This was an open-label, observational, prospective study in which the eligible patients were newly treated with indacaterol (150 or 300 µg) in its approved indication according to the routine medical practice, over a 12- or 24-week treatment period, as applicable. In this PMS study; recruitment of more than 3,000 patients is planned until 2016 according to the Korean Health Authority regulatory guidelines. The study was designed and conducted in accordance with the guidelines for Good Pharmaco-epidemiology Practices and is in compliance with the ethical principles stated in the Declaration of Helsinki. The study protocol was approved by the institutional review board of each study center.
The inclusion criteria of subjects were physician-diagnosed COPD patients aged 18 and over, prescribed with indacaterol either 150 or 300 µg for the maintenance treatment. The exclusion criteria were patients with hypersensitivity to indacaterol capsule or any of the ingredients of the capsule, or with lactose intolerance, or with asthma. The written informed consent of patients for participation in this study was directly taken by each investigator. Patients with hypersensitivity to indacaterol capsule or any of the ingredients of the capsule, or with lactose intolerance, or with asthma, were not included in the study.
Safety evaluation was based on the incidence of AEs and serious adverse events (SAEs) during routine drug use. An AE was defined as any unfavourable and unintended sign (including an abnormal laboratory finding), symptom or disease (new disease or worsening of existing disease) occurring after the start of the study medication. Data were collected and recorded, whether volunteered by the subject, discovered by investigator questioning or detected through physical examination, laboratory test or by any other means. Any event was regarded as an SAE, if any one of the following occurred: (1) AE resulting in death/life-threatening condition, (2) required hospitalisation or prolongation of hospitalization, (3) resulted in persistent or significant disability/inability, (4) resulted in a congenital anomaly or a birth defect, and (5) important medical event that jeopardized the subject or may require medical or surgical intervention to prevent one of the outcomes listed above.
Classification of AEs for this study was based on the Medical Dictionary for Regulatory Activities (MedDRA) 16.0 Classification Criteria. Incidences of AEs and SAEs, unexpected AEs and SAEs which were defined as events not listed in the local label were also assessed. During recording of AEs, following information was included type of AE; onset and stop date; severity (mild, moderate, or severe); any SAE occurrence; outcome of AEs; relationship to the study drug; or any concomitant medications, disease, action taken, and treatment given. The severity of AEs was determined as follows: mild, symptom(s) not interfering with the patient's daily activities and continuous treatment with same dose of indacaterol is possible; moderate, symptom(s) were interfering with the patient's daily activities so that the dose decrease of indacaterol or any treatment is needed; and severe, symptom(s) resulting in the patient's inability to undertake daily activities or discontinuation of indacaterol due to AEs. Several factors were considered to check whether there was any relationship with the safety variables. These factors were gender, age, disease duration, history of COPD exacerbation, smoking history, treatment duration, average daily dose of indacaterol, comorbidities, and concomitant drugs or concomitant therapies used.
Effectiveness was evaluated by interviewing the patients by trained and certified physicians and also based on change from baseline in forced expiratory volume in 1 second (FEV1) when the pulmonary function test results were available at site. Change in COPD condition at week 12 or 24 (whichever applicable) was assessed in terms of ‘improved’ (improvement of symptoms) or ‘maintained’ (little change shown from baseline) or ‘worsening’ (symptoms worsened from baseline) or ‘not assessable’ (assessment is not possible because of loss to follow-up or other reasons). Since indacaterol was approved as a maintenance treatment for COPD patients, both ‘improved’ and ‘maintained’ were assessed as being ‘effective.’
For AEs, the number of the subjects and the number of cases of AEs were calculated. The incidence rates and their 95% confidence intervals (CIs) were estimated and analyzed using the chi-square test or Fisher exact test. The number of cases and the percentage of AEs were calculated according to severity, SAEs, outcome, causality with the study drug, actions taken with regard to the study drug, and treatment for AEs. AEs/SAEs whose causality was related to the study drug were referred to as ‘adverse drug reactions.’ Patients excluded from the safety analysis set were those who ‘did not take the study drug’ and those ‘who was lost to follow-up.’
FEV1 values at week 12 or 24 (as applicable) from baseline (before administration) were recorded (if the patients underwent a spirometry test) and the changes before and after study drug administration were analyzed using the paired t test. The effectiveness rate was assessed according to demographic data including key factors such as age, gender, duration of disease, exacerbation history, smoking history, total duration of treatment, total dosage, treatment discontinuation and its reason, concomitant medication use and its type, concomitant treatment and its type. The effectiveness rate and its 95% CIs were then estimated and analyzed using the chi-square test or Fisher exact test. Multivariate logistic regression analysis was performed to find factors affecting effectiveness rate and incidence rate of AEs. All statistical analysis was performed using SAS release version 9.4 (SAS Institute Inc., Cary, NC, USA).
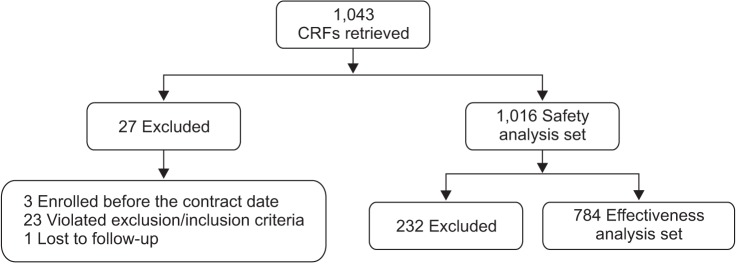
Of the 1,043 enrolled patients, 1,016 patients (97.4%) were included in the safety analysis set. Of these, 784 patients (77.2%) were part of the effectiveness analysis set while 232 patients (22.8%) were excluded due to inability to assess their final effectiveness (i.e., those who did not visit at week 12 and week 24) (Figure 1).
Patient demographics and baseline characteristics are presented in Table 1. The average patient age was 69.1±9.2 years and 87.7% of patients were male. Most patients were followed up at outpatient clinic (97.2%) and 76% of patients had no history of exacerbation in the previous 12 months. Prior to study enrolment, 13.1% and 35.7% of patients were using inhaled corticosteroids (ICS)/LABA and LAMA, respectively. ICS use including ICS/LABA at baseline was 14.6% of patients. Spirometry was available for 476 patients with the mean FEV1 as 1.46±0.47 L. On an average, patients were treated with indacaterol for 150.3±66.1 days. Majority of patients (91.2%) received the daily treatment dose of 150 µg while only 7.6% of the patients received 300 µg.
Overall, 228 patients (22.44%) reported at least one AE with a total of 313 cases of AEs, during this interim analysis. The most common AEs were cough (4.04%) followed by dyspnoea (2.95%), COPD worsening (1.77%), and pneumonia (1.77%). ‘Adverse drug reactions’ occurred in 50 patients (4.92%). Cough (2.76%), drug ineffective (0.89%), and dyspnoea (0.39%) were the most frequent adverse drug reactions in these patients (Table 2).
SAEs were reported by 98 patients (9.65%) with a total of 131 cases during this interim analysis. Of 1,016 patients the most frequent SAEs were dyspnoea (1.87%, 19) followed by pneumonia (1.67%, 17), and COPD worsening (1.38%, 14) (Table 2).
Some of the cases of the reported AEs and SAEs were unexpected, which are defined in the protocol as the events not listed on the local label of the study drug; 13% of patients reported 169 AEs while 8.2% of patients reported 105 SAEs without expectation. SAE suspected to be related to the study medication was orthostatic hypotension, which was reported by one patient. Ninety patients in this study population were hospitalized and nine patients died during the treatment period. All death was assessed to be not related to study medication as determined by the investigator.
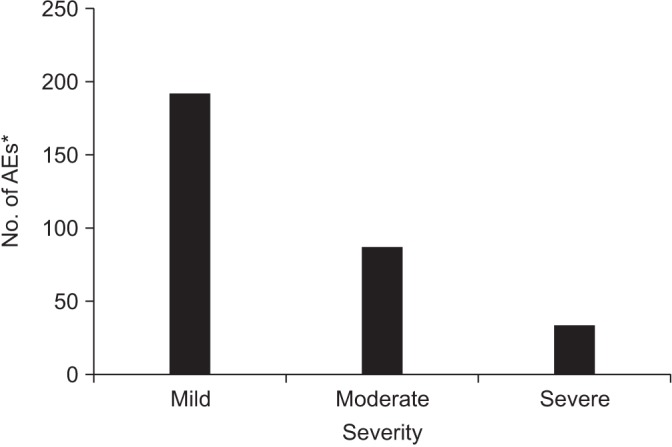
Among 313 reported AEs, majority of them were ‘mild’ (61.3%) while the proportion of ‘moderate’ and ‘severe’ cases was lower (27.8% and 10.9%, respectively) (Figure 2). The AEs could either be resolved completely (67.1%), or on recovery (20.1%) while some of them were not resolved (10.5%), resolved with sequela (0.7%) or remained worsened (1.6%). For the management of AEs, the most common action taken with the study drug was no change (77.6%) followed by permanently discontinued (20.8%), temporarily discontinued (1.3%), and dosage increased (0.3%).
There were no differences in the incidence rate of AEs either by gender or age. However, a statistically significant difference in the incidence rate was observed for patients who suffered from COPD for more than 3 years as compared with patients who had the disease for less than 1 year (28.41% vs. 19.76%, respectively; p=0.045). In addition, an analysis of the incidence of AEs based on the presence and absence of COPD exacerbations revealed that 35.25% (86/244) of the patients with AEs had experienced a COPD exacerbation, while 18.39% (142/772) had not (p<0.001) (Table 3). Multivariable analysis showed that COPD exacerbation history (relative ratio [RR], 1.88; p=0.001), previous medical history (RR, 2.05; p<0.001), comorbidities (RR, 2.19; p=0.002), concomitant drugs (RR, 3.45; p=0.01), and concomitant therapies used (RR, 7.91; p=0.003) were the factors that affects the incidence rate of AEs (Table 4).
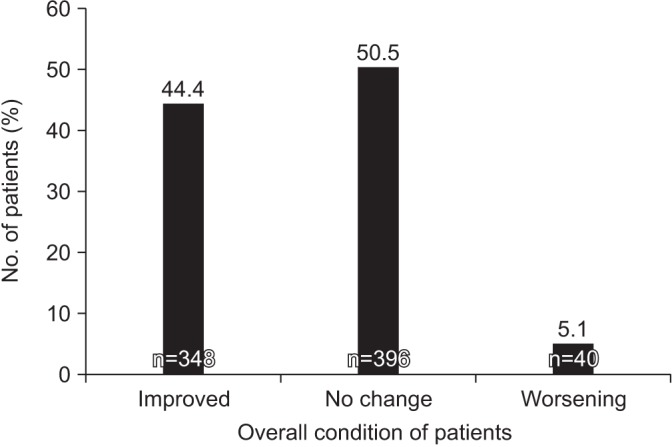
Physicians subjectively assessed the patient's overall status based on the changes in FEV1 and symptom improvement at week 12 or 24, through proportions of ‘improved’, ‘maintained’ and ‘worsening’ cases. Most of the patients either showed an improvement in their COPD condition or the condition was maintained, while only 5.1% patients exhibited worsening of symptoms (Figure 3).
Mean value of FEV1 collected from 476 patients before drug administration was 1.46±0.47 L and 149 patients (31.3%) repeated pulmonary function test at week 12. The mean value of FEV1 was statistically increased by 0.05±0.23 L (p=0.007) (Table 5).
Within several factors considered for the effectiveness assessments, the results showed that there was a statistically significant relationship between the effectiveness rate and three key factors: COPD exacerbation history (p=0.004), smoking history (p=0.048), and average daily dose of the study drug (p=0.003) (Table 6). In multivariable analysis, COPD exacerbation history (p=0.011) and average daily dose of study drug (p=0.003) were independent factors that affect the effectiveness rate (Table 4).
This interim analysis of PMS study was conducted in order to demonstrate the safety and effectiveness of indacaterol (150 µg and 300 µg) in COPD patients in South Korea. Both the doses (150 µg and 300 µg) were well tolerated with a lower incidence of AEs and causality related to the study drug. The overall condition of patients included in this study, based on physician assessment, was either improved or maintained; only few patients (5.1%) exhibited worsening of symptoms. AEs reported by patients were mostly mild or moderate. Moreover, the incidence rate of unexpected AEs reported was also less and could be resolved, reflecting a good safety profile of indacaterol in real-world setting. In most of clinical trials, it has been shown that indacaterol is generally safe and well tolerated in patients with moderate-to-severe COPD4. In a pooled analysis of data from all completed indacaterol clinical trials of at least 12-week duration in patients with moderate-to-severe COPD, no significant safety issues were observed with indacaterol use9. Here, the most common events were COPD worsening, nasopharyngitis, headache, cough, and upper respiratory tract infection. In this analysis, the mean percentage of attended visits at which patients experienced cough after inhalation of indacaterol ranged from 14.1% to 18.4% across the indacaterol dose groups (75, 150, 300, and 600 µg) and for the majority of these patients, the cough started within 15 seconds of inhalation and lasted ≤15 seconds. Long-term studies, like the INDORSE study, have shown that the tolerability profile of indacaterol in patients with moderate-to-severe COPD was similar to that reported in short-term trials, with the majority of AEs being mild-to-moderate in severity10. The incidence of AEs and SAEs were 76%, 77% and 10.4%, 12.3% in indacaterol treatment groups with 150 µg and 300 µg, respectively. However, the incidence rate of AEs in our study was 22.4%, much lower than previous study. We assume there is likelihood that AEs were less frequently reported in a real-world clinical setting of South Korea with short study period compared to phase III clinical trials. Also, the incidence of AEs with indacaterol therapy in Asian patients with moderate-to-severe COPD was found to be similar as in the Caucasian population7. Recently, a post-marketing study conducted in Japan to evaluate the efficacy of indacaterol, 150 µg on QoL (modified Medical Research Council, dyspnoea scale and COPD Assessment Test) and pulmonary function, showed that indacaterol was effective and well tolerated as a bronchodilator for management of patients with COPD11. Real-world studies provide a better picture of effectiveness and safety of an approved drug in clinical-practice across diverse population. In one such study (INFLOW) where various bronchodilators including indacaterol were evaluated in COPD patients across Middle East, Asia and South Africa, an improvement in health status was reported and the bronchodilators were well-regarded by both the physicians as well as patients12. Since this is an observational study in which there are no placebo or comparators, one must be careful in predicting the actual factors which may influence the AEs and the effectiveness of the study drug. In this real-world study, inherent reporting bias, stability of concomitant medications, and physician's subjective evaluation at different centers could be limiting factors. However, low number of reported AE and SAEs along with proven effectiveness of indacaterol in this study re-establishes the outcomes of clinical trials, indicating indacaterol as an effective and well-tolerated bronchodilator option for the maintenance treatment of COPD patients.
Acknowledgments
The authors thank Parveen Sarkari (Novartis) for assistance in the preparation of this manuscript. This study was funded by Novartis Korea Ltd., Seoul, South Korea.
Notes
Conflicts of Interest: Y.-M.O. has received honoraria/consulting fees for Dong-Wha, MSD Korea, AstraZeneca Korea, GlaxoSmithKline Korea, Novartis, Boehringer-Ingelheim Korea in the recent 3 years.
All investigators (H.-K.Y., H.-R.K., Y.S.C., K.-C.S., and Y.-M.O.) received fees from Novartis for conducting the study. S.K. is a Novartis employee.
References
1. Gold Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. Gold Initiative for Chronic Obstructive Lung Disease; 2016. cited 2016 Jan 12. Available from: http://www.goldcopd.org.
2. Park H, Jung SY, Lee K, Bae WK, Lee K, Han JS, et al. Prevalence of chronic obstructive lung disease in Korea using data from the fifth Korea national health and nutrition examination survey. Korean J Fam Med 2015;36:128-134. PMID: 26019762.




3. Murphy L, Rennard S, Donohue J, Molimard M, Dahl R, Beeh KM, et al. Turning a molecule into a medicine: the development of indacaterol as a novel once-daily bronchodilator treatment for patients with COPD. Drugs 2014;74:1635-1657. PMID: 25212789.



4. McKeage K. Indacaterol: a review of its use as maintenance therapy in patients with chronic obstructive pulmonary disease. Drugs 2012;72:543-563. PMID: 22356291.


5. Patalano F, Banerji D, D'Andrea P, Fogel R, Altman P, Colthorpe P. Addressing unmet needs in the treatment of COPD. Eur Respir Rev 2014;23:333-344. PMID: 25176969.



6. Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;1:CD010139PMID: 25575340.


7. Kinoshita M, Lee SH, Hang LW, Ichinose M, Hosoe M, Okino N, et al. Efficacy and safety of indacaterol 150 and 300 microg in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology 2012;17:379-389. PMID: 22122202.


8. To Y, Kinoshita M, Lee SH, Hang LW, Ichinose M, Fukuchi Y, et al. Assessing efficacy of indacaterol in moderate and severe COPD patients: a 12-week study in an Asian population. Respir Med 2012;106:1715-1721. PMID: 23040786.


9. Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011;6:477-492. PMID: 22003293.



10. Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, et al. Long-term safety and efficacy of indacaterol, a long-acting beta(2)-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011;140:68-75. PMID: 21349928.


11. Ohno T, Wada S, Hanada S, Sawaguchi H, Muraki M, Tohda Y. Efficacy of indacaterol on quality of life and pulmonary function in patients with COPD and inhaler device preferences. Int J Chron Obstruct Pulmon Dis 2014;9:107-114. PMID: 24489464.


12. Juvelekian G, El-Sorougi W, Pothirat C, Yunus F, De Guia T, Kuo HP, et al. A real-world evaluation of indacaterol and other bronchodilators in COPD: the INFLOW study. Int J Chron Obstruct Pulmon Dis 2015;10:2109-2120. PMID: 26491281.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




