 |
 |
| Tuberc Respir Dis > Volume 84(1); 2021 > Article |
|
Abstract
The coronavirus pandemic, known as coronavirus disease 2019 (COVID-19), is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus first identified in patients from Wuhan, China. Since December 2019, SARS-CoV-2 has spread swiftly around the world, infected more than 25 million people, and caused more than 800,000 deaths in 188 countries. Chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) appear to be risk factors for COVID-19, however, their prevalence remains controversial. In fact, studies in China reported lower rates of chronic respiratory conditions in patients with COVID-19 than in the general population, while the trend is reversed in the United States and Europe. Although the underlying molecular mechanisms of a possible interaction between COVID-19 and chronic respiratory diseases remain unknown, some observations can help to elucidate them. Indeed, physiological changes, immune response, or medications used against SARS-CoV-2 may have a greater impact on patients with chronic respiratory conditions already debilitated by chronic inflammation, dyspnea, and the use of immunosuppressant drugs like corticosteroids. In this review, we discuss importance and the impact of COVID-19 on asthma and COPD patients, the possible available treatments, and patient management during the pandemic.
At the end of 2019, an outbreak of an atypical, viral and contagious pneumonia was reported in Wuhan, China, and a novel coronavirus was identified as the causative agent in January 2020 [1]. The novel coronavirus is closer to severe acute respiratory syndrome coronavirus (SARS-CoV)-1 than to Middle East respiratory syndrome coronavirus, which caused two major epidemics in 2002 and 2012, respectively. It has been provisionally named new coronavirus 2019 (nCoV-2019) [2], and then renamed SARS-CoV-2 by the International Virus Taxonomy Committee [3].
Known as coronavirus disease 2019 (COVID-19), the illness was characterized by varying signs and symptoms, ranging from a common infection of the upper respiratory tract to respiratory distress. Person-to-person transmission may occur, mainly via respiratory droplets; however, indirect transmission by droplets or aerosols of infected person deposited on inert surfaces remains uncertain [4]. COVID-19 quickly spread to reach the pandemic stage in March 2020 [5], affecting to date (August 31, 2020) more than 25 million people and causing 847,847 deaths worldwide, according to Johns Hopkins University.
SARS-CoV-2 seems to have preference for elderly populations, males or those with comorbidities. The scientific literature agrees that cardiovascular disease and diabetes are important risk factors for the contraction, morbidity and mortality of COVID-19, but diverge on the role of chronic respiratory diseases specifically between China, the United States and Europe. Chronic lung diseases are usually affected by respiratory infections of viral origin that may have an impact on their development and progression [6,7]. Indeed, chronic respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), have a multifactorial origin and are characterized by chronic inflammation of the airways that induces bronchial hyperresponsiveness and decreased lung function. In addition, infection with respiratory viruses including respiratory syncytial virus, rhinovirus or some coronaviruses (OC43, E229), induces significant physiological changes, mainly via the immune response, and may negatively impact patientsŌĆÖ respiratory tract and lead to exacerbation [8,9].
In this review, we analyze the risk of respiratory diseases during the SARS-CoV-2 pandemic and discuss the impact of COVID-19 on patients with chronic respiratory diseases including asthma and COPD.
COVID-19 is characterized by different symptoms that vary from one individual to another and from one study to another (Table 1) [10-16], including fever, dry cough, fatigue, myalgia, ageusia and anosmia [17], that can progress in the most serious cases to acute respiratory distress [18], specifically in patients with comorbidities [19]. The latter are considered as an important risk factor for the morbidity and mortality of COVID-19 [10]. Indeed, several diseases have been reported with varying frequency in patients infected with SARS-CoV-2 (Table 2) [10,19-29].
This single-stranded RNA virus belongs to the Coronaviridae family of the genus Betacoronavirus [30]. It has round or elliptic forms, with a diameter of approximately 60-140 nm [31] and shares the same life cycle as SARS-CoV-1. It binds specifically to the angiotensin converting enzyme (ACE)-2 and the transmembrane serine protease-2 of the host cell [32,33], via S glycoproteins ŌĆ£spikeŌĆØ present on its surface. The whole forms a complex, which allows the fusion of the two membranes [34] and the release of the viral genome in the cytoplasm [32] where it is translated into two polyproteins (pp1a and pp1ab) responsible for its replication and its transcription [31].
The newly synthesized genomic RNA, the core proteins, and the envelope glycoproteins assemble and form viral particles, contained in Golgi vesicles, which exit the cell by exocytosis to infect other cells and invade the mucous membranes of the airways [35].
Specialized immune cells recognize viral antigens and induce the activation of several signaling pathways and transcription factors [36] which, in turn, stimulate a variety of proinflammatory cytokines involved in the antiviral response by activating the phagocytic cells and mediating adaptive immunity [37,38]. T-helper (Th) lymphocytes, derived from the differentiation of activated CD4+ T lymphocytes, secrete cytokines that stimulate the differentiation of B lymphocytes into anti-SARS-CoV-2 antibody producing plasma cells, and CD8+ T lymphocytes to cytotoxic effector cells capable of eliminating infected cells [39,40], this results in lung lesions visible on computed tomography (CT)-scan [41]. In some patients the increased secretion of several cytokines causes pulmonary hyper-inflammation [20,42]; moreover, SARS-CoV-2 infection induces a decrease in the expression of ACE2, which, in turn, increases angiotensin 2 plasma concentrations that may cause worsening of respiratory symptoms or even acute respiratory distress [43].
Evidence shows that a respiratory virus infection would impact patients with chronic respiratory diseases such as asthma and COPD; hence, lower respiratory infections are considered as important risk factors for exacerbation and hospitalization [44,45]. The World Health Organization (WHO) classifies asthma and COPD as major public health problems, in fact, COPD was the third leading cause of death worldwide in 2016 [46].
The fact is that studies do not show a correlation between having a chronic respiratory disease and contracting SARS-CoV-2 infection, but it represents one of the most reported comorbidities in COVID-19 patients [47]. Based on studies from China, Halpin et al. concluded that the prevalence of chronic respiratory disease in COVID-19 patients was significantly lower than its prevalence in the general population [48]. However, in the United States and Europe, it was at least as high, if not greater, than their prevalence in the general population [21,49,50]. In addition, the reported rates of COPD are significantly higher than those of asthma in China, while the trend is reversed in the United States (Table 3) [10,11,21-25,27,29,50-59]. These prevalence differences could be explained by an underdiagnosis and misreporting of respiratory disease in COVID-19 patients in China compared to the United States and other European countries [50,51], or due to patientsŌĆÖ behavior amid the pandemic in those countries. In addition, a similar trend in the prevalence of the two diseases was observed in the general population. Indeed, in China, the prevalence of COPD was estimated at 8.6% [60] while that of asthma was only 4.2% [61]; however, in the United States the prevalence of COPD was 5.9% [62], compared to 7.7% for asthma [49].
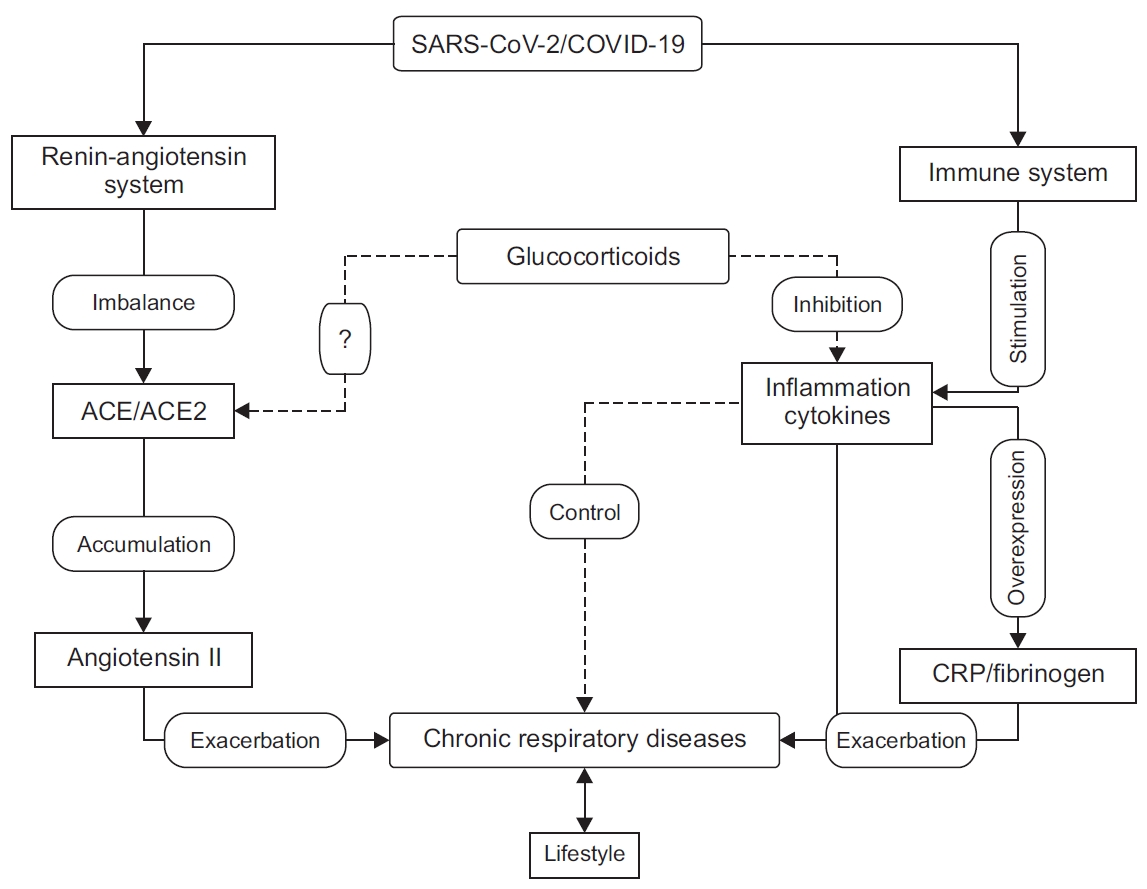
Although SARS-CoV-2 infects healthy people and those with chronic respiratory diseases in the same way, the latter present with more severe symptoms [10,63], higher probability of being admitted to intensive care [64], and a higher mortality rate compared to patients without comorbidities [22]. The mechanisms underlying these differences in the virulence of SARS-CoV-2 between patients with chronic respiratory diseases and patients without comorbidities are still debated by the scientific community, however, some observations give us clues to explore and to elucidate them (Figure 1).
ACE2 is a transmembrane metalloprotease expressed in various tissues, including the heart, intestines, upper and lower airways [65]. This receptor is overexpressed in the epithelium of the bronchioles in COPD patients compared to controls, and could partly explain the severity of COVID-19 in these patients, since overexpression of ACE2 would facilitate virus penetration into the host cells [66]. By contrast, Jackson et al. reported that ACE2 is downregulated in atopic asthmatics after exposure to an allergen and suggested that this may explain the low prevalence of asthma in COVID-19 patients [67]. However, another study showed that there was no significant difference in the level of ACE2 expression throughout the bronchial epithelium in patients with chronic respiratory diseases and healthy controls [68]. Moreover, studies from the U.S. show a significantly higher prevalence of asthma sufferers with COVID-19 [23,24].
On the other hand, ACE is overexpressed in asthma and COPD patients [69]. Moreover, during SARS-CoV-2 infection, ACE2 is negatively regulated [70], leading to an increase in angiotensin 2 plasma concentrations, which are correlated with worsening of respiratory symptoms [71] and a high level of proinflammatory cytokines [72]. In brief, the ACE/ACE2 ratio seems to play an important role in modulating the immune response and the severity of COVID-19, particularly in patients with chronic respiratory diseases.
Infection with SARS-CoV-2 leads to deregulation of some pro-inflammatory cytokine levels [11,42], and other biological parameters, some of which have been suggested to be important markers for predicting the severity of COVID-19. These include, among others, C-reactive protein (CRP), interleukin (IL)-6, lactate dehydrogenase, amyloid A protein, the ratio of neutrophils/lymphocytes, D-dimers and cardiac troponin, whose values are significantly higher in severe COVID-19 patients compared to non-severe patients. However, lymphocyte and platelet counts decrease with the severity of the disease [73]. This deregulation is independent of having a chronic respiratory disease [74], however, the consequences would not be the same for the patients with chronic respiratory disease compared to those without. In fact, the concentration of tumor necrosis factor alpha (TNF-╬▒), which is higher in asthma patients compared to controls before infection [75], is positively correlated with bronchial hyperreactivity [76]. Moreover, a high TNF-╬▒ level is significantly related to a low maximal expiratory volume per second in asthma and COPD patients [77], thus perhaps increasing the risk of having an exacerbation during SARS-CoV-2 infection.
IL-6 is a pleiotropic cytokine involved in several inflammatory processes. It is also involved in modulating the immune response towards a Th-17 profile by initiating the differentiation of naive CD4 T cells into Th-17 pro-inflammatory effector cells, and by inhibiting their conversion into regulatory T cells that have the role of resolving the inflammation [78], thus maintaining the inflammatory state. Furthermore, a high level of IL-6 is linked to a deterioration of lung function [79] and an increase in mortality in COPD patients [80]. This could worsen the symptoms of COPD patients with COVID-19; indeed, COPD has been reported to be an independent risk factor for the severity of COVID-19 [63].
The increased serum level of IL-6 affects the liver and induces an overproduction of CRP and fibrinogen [81]. In COPD patients, these two proteins were found to be significantly higher than in controls [82]. The same trend was reported in asthmatics [83], where CRP level is inversely proportional to the asthma control test score [84]. In addition, increased CRP and fibrinogen concentrations were associated with more exacerbations and higher mortality rate in COPD patients [85]. In asthmatics, the exacerbation was accompanied by an increase of serum CRP level [86].
Although studies do not show a clear link between COVID-19 and exacerbation of chronic respiratory disease, other coronaviruses are risk factors [87], and the involved molecular mechanisms remain unknown. Nevertheless, some biological parameters induced by SARS-CoV-2 may constitute interesting targets to investigate.
COVID-19 causes very pronounced pneumonia [88], in fact, the chest CT scans show unilateral or bilateral ground-glass opacities in almost all patients, including asymptomatic cases. These lung opacities progress to dense lesions at an advanced stage of the disease [10,41]; however, during the first 2 days after the onset of symptoms, the CT scan may be negative [89].
Pneumonia significantly impacts the length of hospital stay and increases the need for artificial respiration in COPD patients [90]. In asthmatics, pneumonia can increase the risk of being hospitalized or of being admitted to the emergency room for exacerbation during the following year after hospital discharge [91]. In addition, pneumoniae are linked to a decrease in the effectiveness of treatments used by asthmatics, specifically ╬▓-mimetics [92].
At present, prevention and protection measures are the only alternatives available to populations since no vaccine is expected for at least a year according to the WHO. A series of hygiene and preventive measures for the general population during the COVID-19 pandemic are published by WHO [93] and should be strictly applied, particularly by patients with chronic respiratory diseases as they are at high risk of developing severe symptoms when contracting SARS-CoV-2 infection.
At this time, no specific treatment for COVID-19 is available; however, repositioning for some existing drugs is being tested around the world. Indeed, in China, the National Health Commission has advocated the use of existing antivirals: interferon-╬▒ commonly used to treat hepatitis-C, the lopinavir/ritonavir (Lpv/Rtv) combination used in the treatment of human immunodeficiency virus 1, Arbidol used to treat severe cases of influenza, chloroquine (CQ) and its derivatives (hydroxychloroquine [HCQ] and chloroquine-phosphate) known for their efficacy against plasmodium and Remdesivir that has a broad spectrum of antiviral action [94].
In South Korea, Lpv/Rtv was tested successfully on a 54-year-old patient with COVID-19, a significant drop in viral load and an improvement in the patientsŌĆÖ health were noticed [95]. However, a retrospective study suggested that this drug is more effective when combined with Arbidol [96]. This hypothesis was verified in another study where four patients were treated, three of whom were completely cured after 15 days of treatment, the fourth, who was in critical condition on admission, had his stay extended and his condition improved [97].
However, in a comparative study, Cao et al. [98] concluded that there was no significant difference in symptom improvement and death rate in patients with severe disease between a group treated with Lpv/Rtv and another receiving a ŌĆ£standardŌĆØ treatment. This suggests that Lpv/Rtv treatment is only effective when given at an early stage of the disease and in combination with other molecules such as Arbidol.
Remdesivir, an adenosine analogue with a broad antiviral spectrum, has been used to treat COVID-19 in different countries around the world but with controversial outcomes. Indeed, a multi-center study including 53 severe patients from three countries, 30 of whom required mechanical ventilation, reported that 68% of patients treated with remdesivir saw their condition improve after 18 days of hospitalization with a mortality rate of 13% [99]. Conversely, a randomized clinical trial conducted in China on 237 patients with severe forms of COVID-19 did not show a significant difference between the group treated with remdesivir and the control group [100]. However, another randomized clinical trial conducted in the United States on more than 1,000 COVID-19 patients, demonstrated better efficacy, since the administration of remdesivir significantly reduced the time of hospitalization and the mortality rate [101].
CQ is the controversial ŌĆ£moleculeŌĆØ that is still in debate today. In fact, this molecule has proven its antiviral power in vitro against SARS-CoV-2 [102]. CQ may be a drug of choice since it has few side effects and is well known by the scientific community as it has been prescribed for 70 years to treat malaria, rheumatoid arthritis, and certain forms of lupus [103]. Some researchers recommended its use for any intracellular infection, whether viral, fungal or bacterial [104]. However, clinical trials are needed to prove its effectiveness against SARS-CoV-2. Despite the fact that CQ was effective in vitro against dengue fever, all the following clinical trials were unsuccessful as it failed to reduce the hospitalization time [105,106].
In the current global health crisis, Algeria, among other worldwide countries, has advocated the use of CQ as a first-line treatment for patients with a moderate or severe form of COVID-19 [107]. This decision was motivated by the result of a preliminary clinical trial conducted in China in which nearly 100 COVID-19 patients were subjected to CQ treatment across 10 hospital centers; this treatment significantly reduced the hospital length of stay and the intensity of symptoms compared to the control group [108]. In addition, a study conducted in France on 36 patients with COVID-19, of whom 20 received treatment with HCQ with or without azithromycin, and 16 received symptomatic treatment, showed that after 6 days all of the patients treated with the HCQ-azithromycin combination were cured compared to 57% of patients treated with HCQ alone and 12% of patients in the control group [109]. Another study conducted by the same team on more than 1,000 COVID-19 patients demonstrated remarkable effectiveness, as nearly 99% of patients were cured after 15 days of hospitalization, with a death rate of 0.75% [110]. However, cliniciansŌĆÖ opinions remain divided on this matter and the main criticisms against these last two studies are the limited sample size for the first study, and the absence of a control group for the second study. In addition, the results of the British clinical trial ŌĆ£RecoveryŌĆØ, which is the first large and controlled randomized clinical trial are disappointing; indeed, HCQ did not reduce the time of hospitalization nor the mortality rate in COVID-19 patients during the 28-days hospital stay compared to the control group [111].
Facing an important variability in the symptoms and in the severity of SARS-CoV-2 infection, health practitioners are challenged to shorten the hospital stay or to save the patientsŌĆÖ lives by using all known and available therapies. Indeed, some studies suggest the use of glucocorticoids (GCs), while others prefer immunotherapy, including anti-IL-6 monoclonal antibodies and the serum of convalescent patients [112] to modulate the excessive or insufficient immune response. Moreover, others suggest the use of bronchodilators for the treatment of acute respiratory distress in critically ill COVID-19 patients [113].
Apart from the Lpv/Rtv combination that presents an increased risk of adrenal insufficiency due to the simultaneous use of inhaled GCs and Ritonavir [114,115], studies do not show any particular adverse effects of these treatments on COVID-19 patients with asthma or COPD. In addition, there are currently no specific guidelines on the therapies to use for COVID-19 patients with chronic respiratory comorbidities, but their management during this pandemic was the subject of several recommendations from public health organizations [116].
Asthmatics and COPD patients receive a basic treatment to control their disease in which inhaled or systemic GCs were used to reduce inflammation and bronchial hyperresponsiveness. However, administration of GCs at high doses (>150 mg/day) would increase the mortality rate in COVID-19 patients [52], but at lower doses studies have shown no difference in morbidity and mortality from COVID-19 between patients receiving GCs and control patients [117,118]. Other studies have suggested that they may even have a protective effect since prolonged use of GCs negatively regulates the expression of ACE2 [33]; moreover, ciclesonide, one of the GCs used in the treatment of chronic respiratory diseases, was able to inhibit SARS-CoV-2 replication in vitro [119].
Another study conducted with three COVID-19 patients over the age of 65, two of whom required oxygen therapy, showed encouraging results since the administration of this GC by inhalation improved the general health condition of the patients and significantly reduced viral load [120]. It is therefore recommended that asthmatics and COPD patients should continue their usual treatments with inhaled GCs in order to better control their disease, as this would allow them to reduce the frequency of exacerbations in the event of a viral infection with SARS-CoV-2 [121].
Additionally, special attention should be paid to the treatment of comorbidities, including allergic rhinitis and cardiovascular disease, as well as the management of exacerbations in asthma and COPD during the pandemic. In fact, allergic rhinitis is reported in over 78% of asthmatics and is considered to be a risk factor for the onset and exacerbation of asthma [122]. Moreover, the lack of control of allergic rhinitis is correlated with that of asthma [123]. Therefore, monitoring the treatment for allergic rhinitis at prescribed doses is strongly recommended, in parallel with that of asthma, specifically GCs administered by the nasal route [124] or allergen immunotherapy. Hence, for any new prescription of the latter, the sublingual method should be preferred to subcutaneous injection to avoid repeated medical visits [125].
Cardiovascular disease, including high blood pressure, is a very common comorbid condition in COPD patients, with prevalence rates up to 70% [126]. In addition, treatments used for cardiovascular disease do not have any adverse effects on COPD patients; they may even have some benefit in controlling the disease [127]. Consequently, the French and European hypertension societies recommend continuing the treatments based on the inhibitors of the renin-angiotensin system and antagonists of angiotensin receptors during the COVID-19 pandemic [128].
Finally, regarding the exacerbation of asthma and COPD, it would seem imperative to ensure that the patient masters the technique of inhaled bronchodilators to avoid hospitalization during this pandemic [129].
Improving current knowledge and understanding the impact of SARS-CoV-2 infection on respiratory diseases will help to improve the management of COVID-19 patients with chronic respiratory diseases. The prevalence of those comorbidities in COVID-19 patients differs between Chinese studies and European and American studies. Indeed, the reported rates of COPD and asthma in COVID-19 patients are significantly lower in China compared to the United States or Europe.
The impact of viral infections on chronic respiratory disease is well known; however, that of SARS-COV-2 remains controversial and may be underestimated or not fully understood. In addition, it appears that patients with respiratory diseases, including asthma and COPD, would not react in the same way to COVID-19. Indeed, infection with SARS-CoV-2 causes pneumonia and produces an imbalance in the ACE/ACE2 ratio and some biological parameters known to have negative effects on asthmatics and COPD patients that could worsen their symptoms and may increase their risk of exacerbation.
There is currently no specific treatment for COVID-19, but clinical trials of existing drugs are being carried out around the world. In this context, special attention should be paid to asthmatics and COPD patientsŌĆÖ treatments to avoid any risk of undesirable drug interactions, treatment interruption or overdose.
In conclusion, the evidence we have supports the idea that COVID-19 could have a more pronounced impact on asthmatics and COPD patients compared to those without comorbidities. However, further studies are needed to better elucidate the impact of this new infection on respiratory diseases and to better assess the potential risk for these patients.
Notes
Figure┬Ā1.
Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on chronic respiratory diseases. SARS-CoV-2 activates the hostŌĆÖs immune system and stimulates the production of pro-inflammatory cytokines, which may increase the risk of exacerbations of chronic respiratory diseases directly or indirectly by stimulating the production of C-reactive protein (CRP), fibrinogen, and cytokines. SARS-CoV-2 also affects the renin-angiotensin-aldosterone system by negatively regulating the expression of angiotensin-converting enzyme 2 (ACE2), which causes the accumulation of angiotensin 2 in the lung tissue and may exacerbate or worsen the symptoms of chronic respiratory diseases. Glucocorticoids (GCs) modulate the immune response and reduce inflammation and help to control chronic respiratory disease. The effect of GCsŌĆÖ on ACE2 expression remains debated; their role in preventing SARS-CoV-2 infection has not been fully investigated. COVID-19: coronavirus disease 2019.
Table┬Ā1.
Frequency of signs and symptoms (%) in adult COVID-19 patients at admission
| Study | No. | Fever | Cough | Fatigue/myalgia | Sore throat | Dyspnea | Headache | Diarrhea |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. [10] | 140 | 91.7 | 75 | 75* | - | 36.7 | - | 12.9 |
| Wan et al. [12] | 135 | 88.9 | 76.5 | 32.5* | 17.7 | 13.3 | 32.5 | 13.3 |
| Wang et al. [11] | 69 | 87 | 55 | 42/30 | - | 29 | 14 | 14 |
| Han et al. [13] | 108 | 87 | 60 | 39/11 | 13 | - | 13 | 14 |
| Qian et al. [14] | 91 | 71.43 | 60.44 | 43.96/5.49 | - | 3.3 | 7.69 | 23.08 |
| Young et al. [15] | 18 | 72 | 83 | - | 61 | - | - | 17 |
| Xu et al. [16] | 90 | 78 | 63 | 21/28 | 26 | - | 4 | 6 |
Table┬Ā2.
Frequency of comorbidities in COVID-19 patients
| Study | No. | Comorbidities (%) | Countries | Average age (yr) |
Comorbidities (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | Diabetes | CVD | Obesity | Tumors | Neuropathology | Chronic Hepatitis | Chronic renal failure | Immunodeficiency | |||||
| Chen et al. [25] | 99 | 51 | China | 55.5 | - | - | 40 | - | 1 | - | - | - | - |
| Garg et al. [24] | 159 | - | USA | - | 49.7 | 28.3 | 27.8 | 48.3 | - | 14 | - | 13.1 | 9.6 |
| Guan et al. [22] | 1,590 | 25.1 | China | 48.9 | 16.9 | 8.2 | 53.7 | - | 1.1 | - | - | - | 0.2 |
| Grasselli et al. [26] | 1,043 | 68 | Italy | 63 | 49 | 17 | 21 | - | 8 | - | 3 | 3 | - |
| Huang et al. [20] | 41 | 32 | China | 49 | 15 | 20 | 15 | - | 2 | - | 2 | - | - |
| Liu et al. [27] | 137 | 19.7 | 57 | 9.5 | 10.2 | 7.3 | - | 1.5 | - | - | - | ||
| Richardson et al. [21] | 5,700 | - | USA | 63 | 56.6 | 33.8 | - | 41.7 | - | - | - | - | - |
| Wang et al. [19] | 138 | 46.4 | China | 56 | 31.2 | 10.1 | 14.5 | - | 7.2 | - | 2.9 | 2.9 | - |
| Yang et al. [28] | 1,576 | - | 49.6 | 21.1 | 9.7 | 8.4 | - | - | - | - | - | - | |
| Zhang et al. [10] | 140 | 63.4 | 57 | 30 | 12.1 | - | - | - | - | - | - | - | |
| Gold et al. [23] | 305 | - | USA | 60 | 67.5 | 39.7 | 25.6 | 12.7 | 3.9 | 12.5 | 2.3 | 10.5 | - |
| CDC COVID-19 Response Team [29] | 345 | 23 | 11 | 31.25 | - | - | - | - | - | 2.9 | |||
Table┬Ā3.
Prevalence of chronic respiratory disease in COVID-19 patients
| Study | No. | Country | Age groups (yr) |
Respiratory disease (%) |
||
|---|---|---|---|---|---|---|
| All | Asthma | COPD | ||||
| Guan et al. [22] | 1,590 | China | All | 1.5 | - | 1.5 |
| Li et al. [52] | 548 | China | Adults | 4 | 0.9 | 3.1 |
| Li et al. [53] | 25 | China | All | 20 | - | 20 |
| Lian et al. [54] | 788 | China | All | 0.38 | - | 0.38 |
| Liu et al. [27] | 137 | China | 20-83 | 1.5 | - | 1.5 |
| Wang et al. [55] | 339 | China | >60 | 6.2 | - | 6.2 |
| Zhang et al. [56] | 290 | China | All | 2.4 | 0.3 | 2.1 |
| Zhang et al. [10] | 140 | China | 25-87 | 1.4 | - | 1.4 |
| Du et al. [57] | 85 | China | All | 2.4 | - | 2.4 |
| Chen et al. [25] | 99 | China | 21-82 | 1 | - | - |
| Wang et al. [11] | 69 | China | All | 9 | 3 | 6 |
| Richardson et al. [21] | 5,700 | USA | All | 16.14 | 9 | 5.4 |
| Gold et al. [23] | 305 | USA | Adults | 20.3 | 10.5 | 5.2 |
| CDC COVID-19 Response Teamm [29] | 345 | USA | <18 | 11.6 | - | - |
| CDC COVID-19 Response Team [58] | 647 | USA | >18 | 9.2 | - | - |
| Garg et al. [24] | 159 | USA | All | 34.6 | 17 | 10.7 |
| Docherty et al. [50] | 17,634 | UK | All | 32.14 | 14.5 | - |
| Dreher et al. [59] | 50 | Germany | All | 50 | 12 | 22 |
| Cariou et al. [51] | 1,278 | France | All | 10.4 | - | 10.4 |
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-33.



2. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924.



3. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536-44.




4. Conti P, Gallenga CE, Tete G, Caraffa A, Ronconi G, Younes A, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents 2020;34:333-8.

5. WHO Director-GeneralŌĆÖs opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. Geneva: World Health Organization; 2020 [cited 2020 Apr 22]. Available from: https://www.who.int/fr/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
6. Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, et al. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 2005;50:322-30.


7. Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations: a GA(2) LEN-DARE systematic review. Allergy 2011;66:458-68.


8. Silva RC, Couceiro JN, Camara FP, Valle S, Santos N. Asthma exacerbation and viral infection in adult patients, Brazil. Braz J Infect Dis 2015;19:446-8.



9. Kherad O, Bridevaux PO, Kaiser L, Janssens JP, Rutschmann O. Viral infection in acute exacerbation of COPD. Rev Med Suisse 2011;7:2222-6.

10. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730-41.


11. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:769-77.


12. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020;92:797-806.



13. Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR Am J Roentgenol 2020;215:338-43.


14. Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 2020;113:474-81.



15. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323:1488-94.



16. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 2020;47:1275-80.



17. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683-90.


19. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9.



20. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.



21. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020;323:2052-9.



22. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547.



23. Gold JA, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 ŌĆö Georgia, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:545-50.



24. Garg S, Kim L, Whitaker M, OŌĆÖHalloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458-64.



25. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13.



26. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574-81.



27. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025-31.



28. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91-5.



29. CDC COVID-19 Response Team. Coronavirus disease 2019 in children ŌĆö United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422-6.



30. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953-66.


31. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus [Internet]. Treasure Island: StatPearls Publishing; 2020 [cited 2020 Mar 25]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK554776/.
32. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci U S A 2004;101:4240-5.



33. Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med 2020;202:83-90.



34. Izaguirre G. The proteolytic regulation of virus cell entry by furin and other proprotein convertases. Viruses 2019;11:837.



35. Perrier A, Bonnin A, Desmarets L, Danneels A, Goffard A, Rouille Y, et al. The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J Biol Chem 2019;294:14406-21.



36. Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol 2020;30:e2107.


37. Deng X, van Geelen A, Buckley AC, OŌĆÖBrien A, Pillatzki A, Lager KM, et al. Coronavirus endoribonuclease activity in porcine epidemic diarrhea virus suppresses type I and type III interferon responses. J Virol 2019;93:e02000-18.



38. Yang CH, Li K, Pfeffer SR, Pfeffer LM. The Type I IFN-Induced miRNA, miR-21. Pharmaceuticals (Basel) 2015;8:836-47.



39. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2.



40. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 2020;92:1518-24.



41. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425-34.



42. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020;11:827.



43. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318:H1084-90.



44. Hewitt R, Farne H, Ritchie A, Luke E, Johnston SL, Mallia P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther Adv Respir Dis 2016;10:158-74.


45. Liao H, Yang Z, Yang C, Tang Y, Liu S, Guan W, et al. Impact of viral infection on acute exacerbation of asthma in outpatient clinics: a prospective study. J Thorac Dis 2016;8:505-12.



46. Top 10 causes of death [Internet]. Geneva: World Health Organization; 2018 [cited 2019 Nov 27]. Available from: http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/.
47. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020;8:e35.


48. Halpin DM, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med 2020;8:436-8.



49. Most recent national asthma data [Internet]. Atlanta: Centers for Disease Control and Prevention; 2020 [cited 2020 May 8]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
50. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985.



51. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500-15.



52. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146:110-8.



53. Li YK, Peng S, Li LQ, Wang Q, Ping W, Zhang N, et al. Clinical and transmission characteristics of COVID-19: a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci 2020;40:295-300.



54. Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis 2020;71:740-7.


55. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639-45.



56. Zhang JJ, Cao YY, Dong X, Wang BC, Liao MY, Lin J, et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy 2020;75:1809-12.


57. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med 2020;201:1372-9.



58. CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020;69:382-6.



59. Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 2020;117:271-8.



60. Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18.


61. Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17.


62. Croft JB, Wheaton AG, Liu Y, Xu F, Lu H, Matthews KA, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease - United States, 2015. MMWR Morb Mortal Wkly Rep 2018;67:205-11.



63. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049-57.



64. Xu G, Yang Y, Du Y, Peng F, Hu P, Wang R, et al. Clinical pathway for early diagnosis of COVID-19: updates from experience to evidence-based practice. Clin Rev Allergy Immunol 2020;59:89-100.



65. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002;532:107-10.


66. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020;55:2000688.



67. Jackson DJ, Busse WW, Bacharier LB, Kattan M, OŌĆÖConnor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol 2020;146:203-6.



68. Li G, He X, Zhang L, Ran Q, Wang J, Xiong A, et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun 2020;112:102463.



69. Dhawale VS, Amara VR, Karpe PA, Malek V, Patel D, Tikoo K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol Appl Pharmacol 2016;306:17-26.


70. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112-6.



71. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875-9.



72. Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 2002;(82):S12-22.


73. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19: a systematic review. Life Sci 2020;254:117788.



74. Manthei DM, Schwantes EA, Mathur SK, Guadarrama AG, Kelly EA, Gern JE, et al. Nasal lavage VEGF and TNF-alpha levels during a natural cold predict asthma exacerbations. Clin Exp Allergy 2014;44:1484-93.



75. Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, et al. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy 1991;21:745-50.


76. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 1995;152:76-80.


77. Huang AX, Lu LW, Liu WJ, Huang M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit 2016;22:2800-8.



78. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235-8.


79. Garth J, Barnes JW, Krick S. Targeting cytokines as evolving treatment strategies in chronic inflammatory airway diseases. Int J Mol Sci 2018;19:3402.



80. Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1065-72.


81. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145-56.



82. Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005;128:1995-2004.



83. Kasayama S, Tanemura M, Koga M, Fujita K, Yamamoto H, Miyatake A. Asthma is an independent risk for elevation of plasma C-reactive protein levels. Clin Chim Acta 2009;399:79-82.


84. Kilic H, Karalezli A, Hasanoglu HC, Erel O, Ates C. The relationship between hs-CRP and asthma control test in asthmatic patients. Allergol Immunopathol (Madr) 2012;40:362-7.


85. Fermont JM, Masconi KL, Jensen MT, Ferrari R, Di Lorenzo VAP, Marott JM, et al. Biomarkers and clinical outcomes in COPD: a systematic review and meta-analysis. Thorax 2019;74:439-46.



86. Manuyakorn W, Mairiang D, Sirachainan N, Kadegasem P, Kamchaisatian W, Benjaponpitak S, et al. Blood coagulation and asthma exacerbation in children. Int Arch Allergy Immunol 2016;170:75-83.


87. Zheng XY, Xu YJ, Guan WJ, Lin LF. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol 2018;163:845-53.



88. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 2020;214:1072-7.


89. Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for radiologists on COVID-19: an update-Radiology Scientific Expert Panel. Radiology 2020;296:E113-4.


90. Andreassen SL, Liaaen ED, Stenfors N, Henriksen AH. Impact of pneumonia on hospitalizations due to acute exacerbations of COPD. Clin Respir J 2014;8:93-9.


91. Pelton SI, Shea KM, Bornheimer R, Sato R, Weycker D. Pneumonia in young adults with asthma: impact on subsequent asthma exacerbations. J Asthma Allergy 2019;12:95-9.


92. Rueter K, Bizzintino J, Martin AC, Zhang G, Hayden CM, Geelhoed GC, et al. Symptomatic viral infection is associated with impaired response to treatment in children with acute asthma. J Pediatr 2012;160:82-7.


93. Coronavirus disease (COVID-19) advice for public [Internet]. Geneva: World Health Organization; 2020 [cited 2020 Apr 21]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
94. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020;14:58-60.


95. Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of Lopinavir/Ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 2020;35:e79.


96. Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus sisease 2019: a retrospective cohort study. J Infect 2020;81:e1-e5.

97. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 2020;14:64-8.


98. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe COVID-19. N Engl J Med 2020;382:1787-99.


99. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of Remdesivir for patients with severe COVID-19. N Engl J Med 2020;382:2327-36.


100. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569-78.



101. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19: preliminary report. Reply. N Engl J Med 2020;383:994.

102. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269-71.



103. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care 2020;57:279-83.



104. Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 2007;30:297-308.



105. Borges MC, Castro LA, Fonseca BA. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz 2013;108:596-9.



106. Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 2010;4:e785.



107. Algerian Ministry of Health. Coronavirus infection threat preparedness and response plan [Internet]. Algiers: Algerian Ministry of Health; [cited 2020 Mar 31]. Available from: http://www.sante.gov.dz/prevention/.
108. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72-3.


109. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56:105949.



110. Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of 1061 COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 2020;35:101738.



111. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 ŌĆö RECOVERY Trial [Internet]. Oxford: Nuffield Department of Population Health; 2020 [cited 2020 Jun 7]. Available from: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19.
112. AminJafari A, Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int Immunopharmacol 2020;83:106455.



113. Elbeddini A, Yeats A. Amid COVID-19 drug shortages: proposed plan for reprocessing and reusing salbutamol pressurized metered dose inhalers (pMDIs) for shared use. Drugs Ther Perspect 2020;1-3.

114. Zubillaga I, Frances C, Nicolau J, Homar F, Masmiquel L. Adrenal insufficiency and exogenous CushingŌĆÖs syndrome in a patient receiving inhaled fluticasone and ritonavir. Endocrinol Diabetes Nutr 2017;64:338-9.


115. Blondin MC, Beauregard H, Serri O. Iatrogenic Cushing syndrome in patients receiving inhaled budesonide and itraconazole or ritonavir: two cases and literature review. Endocr Pract 2013;19:e138-41.


116. Coronavirus disease 2019 (COVID-19) [Internet]. Atlanta: Centers for Disease Control and Prevention; 2020 [cited 2020 Jun 3]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html.
117. Nasim S, Kumar S, Azim D, Ashraf Z, Azeem Q. Corticosteroid use for 2019-nCoV infection: a double-edged sword. Infect Control Hosp Epidemiol 2020;41:1244-5.


118. Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020;81:147-78.

119. Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. Preprint at http://biorxiv.org/lookup/doi/10.1101/2020.03.11.987016 (2020).

120. Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 2020;26:625-32.


121. Rothuizen LE, Livio F, Buclin T. Drugs that aggravate the course of COVID-19: really ? Rev Med Suisse 2020;16:852-4.

122. Casale TB, Dykewicz MS. Clinical implications of the allergic rhinitis-asthma link. Am J Med Sci 2004;327:127-38.


123. ARIA [Internet]. Woluwe-Saint-Lambert: EUFOREA; 2020 [cited 2020 Jun 3]. Available from: https://www.euforea.eu/aria.
124. Bousquet J, Akdis C, Jutel M, Bachert C, Klimek L, Agache I, et al. Intranasal corticosteroids in allergic rhinitis in COVID-19 infected patients: an ARIA-EAACI statement. Allergy 2020;75:2440-4.


125. Scadding GK, Hellings PW, Bachert C, Bjermer L, Diamant Z, Gevaert P, et al. Allergic respiratory disease care in the COVID-19 era: a EUFOREA statement. World Allergy Organ J 2020;13:100124.



126. Ding B, Kallenbach L, Slipski L, Wilk A, OŌĆÖBrien D, Guranlioglu D. Patient characteristics and healthcare resource utilization among patients with COPD new to LAMA/LABA fixed-dose combination greatment in US-based real-world practice. Int J Chron Obstruct Pulmon Dis 2020;15:775-86.



127. Recio Iglesias J, Diez-Manglano J, Lopez Garcia F, Diaz Peromingo JA, Almagro P, Varela Aguilar JM. Management of the COPD patient with comorbidities: an experts recommendation document. Int J Chron Obstruct Pulmon Dis 2020;15:1015-37.



128. Ssieh RM. European Hypertension Society (ESH) statement on hypertension, regarding renin angiotensin system blockers and COVID-19 disease caused by the SARS-CoV-2 coronavirus [Internet]. Paris: French Society of Hypertension; 2020 [cited 2020 May 29]. Available from: http://www.sfhta.eu/?p=6670.
-
METRICS

- ORCID iDs
-
Belaid Bouazza

https://orcid.org/0000-0002-3958-3667 - Related articles
-
What Single Cell RNA Sequencing Has Taught Us about Chronic Obstructive Pulmonary Disease
Economic Burden of Chronic Obstructive Pulmonary Disease: A Systematic Review
Proposed Etiotypes for Chronic Obstructive Pulmonary Disease: Controversial Issues


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



