 |
 |
| Tuberc Respir Dis > Volume 83(3); 2020 > Article |
|
Abstract
Background
Methods
Results
Notes
AuthorsŌĆÖ Contributions
Conceptualization: Oh YM. Methodology: Oh YM. Formal analysis: Kim HK. Data curation: Lee H, Choi H, Kim SH, Lee JS, Lee SW, Lee JH. Software: Lee H, Choi H, Kim SH, Lee JS, Lee SW, Lee JH. Validation: Kim HK. Investigation: Kim HK. Writing - original draft preparation: Kim HK, Kim SH. Writing - review and editing: Lee H, Choi H, Lee JS, Lee SW, Lee JH, Oh YM. Approval of final manuscript: all authors.
Acknowledgments
Supplementary Material
Fig.┬Ā1.
Fig.┬Ā2.
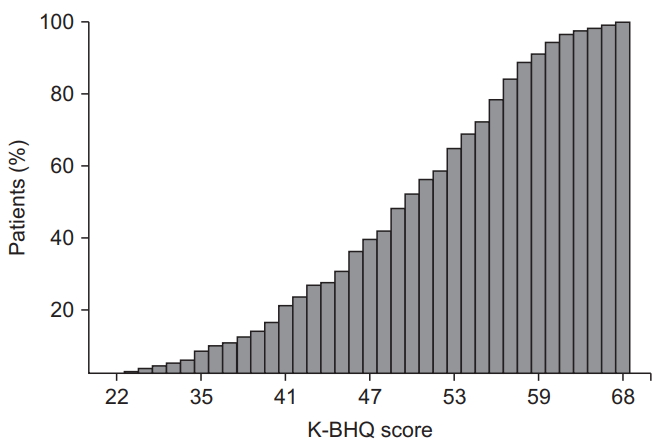
Fig.┬Ā3.

Fig.┬Ā4.

Fig.┬Ā5.

Fig.┬Ā6.

Table┬Ā1.
| Characteristic | Value (n=126) |
|---|---|
| Age, yr | 64.3┬▒9.7 |
| Female sex | 67 (53.2) |
| BMI, kg/m2 | 22.7┬▒3.4 |
| Smoking status | |
| ŌĆāNever | 75 (59.5) |
| ŌĆāEx | 43 (34.1) |
| ŌĆāCurrent | 8 (6.3) |
| Previous pulmonary disease history | |
| ŌĆāTuberculosis | 57 (45.2) |
| ŌĆāMeasles | 30 (23.8) |
| ŌĆāNontuberculous mycobacterium | 13 (10.3) |
| ŌĆāPertussis | 13 (10.3) |
| Prebronchodilator spirometry (n=106) | |
| ŌĆāFEV1 % predicted (L) | 59.6┬▒18.9 (1.62┬▒0.60)* |
| ŌĆāFVC % predicted (L) | 71.7┬▒16.6 (2.59┬▒0.80)* |
| ŌĆāFEV1/FVC % | 54.4┬▒25.5 |
| mMRC dyspnea scale | |
| ŌĆā0 | 35 (28.8) |
| ŌĆā1 | 65 (51.6) |
| ŌĆā2 | 15 (11.9) |
| ŌĆā3 | 9 (7.1) |
| ŌĆā4 | 2 (1.6) |
| Exacerbations in past 12 moŌĆĀ | |
| ŌĆā0 | 82 (65.1) |
| ŌĆā1 | 15 (11.9) |
| ŌĆā2 | 15 (11.9) |
| ŌĆā3 | 5 (4.0) |
| ŌĆā4 | 5 (4.0) |
| ŌĆā5 | 4 (3.2) |
| Exacerbations requiring hospitalization in last 12 mo | |
| ŌĆā0 | 104 (82.5) |
| ŌĆā1 | 14 (11.1) |
| ŌĆā2 | 4 (3.2) |
| ŌĆā3 | 3 (2.4) |
| ŌĆā4 | 1 (0.8) |
| K-CAT score (n=70) | 17.6┬▒9.6 |
| K-BHQ score (n=125) | 49.0┬▒9.1 |
Table┬Ā2.
| K-BHQ correlation coefficient | p-value | |
|---|---|---|
| FEV1, % predicted | r=0.406 | <0.001 |
| FVC, % predicted | r=0.351 | <0.001 |
| Exacerbations in last 12 mo | ||
| ŌĆāAny | Žü=-0.245 | 0.006 |
| ŌĆāMild to moderate* | Žü=-0.116 | 0.198 |
| ŌĆāSevereŌĆĀ | Žü=-0.303 | 0.001 |
| mMRC grade | Žü=-0.409 | <0.001 |
| K-CAT score | r=-0.656 | <0.001 |
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hyun Kuk Kim

https://orcid.org/0000-0002-9360-3956Yeon-Mok Oh

https://orcid.org/0000-0003-0116-4683 - Funding Information
-
Korean Academy of Tuberculosis and Respiratory Diseases
- Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Supplement
Supplement Print
Print Download Citation
Download Citation



