Introduction
Interstitial lung diseases (ILDs), a group of diseases that has not been actively researched so far and garnered little interest. However, recently, interest in ILDs has been raised, being motivated by cumulative findings from continuous research. Those researches have given us better understanding of the disease. In the meantime, recent development of new treatments for idiopathic pulmonary fibrosis (IPF) have also raised related interest.
With support from the Korean Academy of Tuberculosis and Respiratory Diseases, the guideline committee, which is composed mainly of the Korean ILD Study Group, developed guidelines for the treatment of various interstitial pneumonias, including IPF. Through discussion among the members, we selected key clinical questions that can help clinicians treat patients, and formed a guideline committee from February 2016 to develop an evidence-based guideline. For approximately two years, guidelines were developed through series of respective subcommittee meetings for each disease and validated by the regular full committee meetings on a monthly basis.
This guideline has been established mainly to provide the treatment of ILDs which were, if circumstance allows, based on the outcomes of randomized controlled trials. In addition, for a general overview of various ILDs, a brief introduction, natural history, methods of diagnosis, and treatment for ILDs not being supported by randomized controlled trials were also provided.
1. Objective and subjects of guideline
1) Objective
The primary objective of these guidelines is to help clinicians make diagnosis, decide treatment, and follow-up patients with ILDs.
2) Subjects
The main subjects of these guidelines are the clinicians who care for patients with ILDs in Korea. In addition, it can be used by governmental officers, nurses, medical students, patients, and anyone interested in ILDs.
2. Methods for developing guidelines for treatment
1) Literature review
At first, the members of the committee selected key clinical questions in the treatment of four major ILDs (IPF, idiopathic non-specific interstitial pneumonia [NSIP], cryptogenic organizing pneumonia [COP], and ILD associated with connective tissue disease [CTD]). A search for references concerning the selected key clinical questions was performed using three major databases (MEDLINE, Embase, and Cochrane Library). Search terms were selected by subcommittee members. Searching strategies were decided to increase sensitivity as much as possible and search the references extensively. There were no limitations for publication date or search terms. The design of the study was sometimes limited according to the key question. The members reviewed both original studies and systematic reviews. If there was a well-assessed systematic review, the evidence table up to the search year was accepted and later published literatures concerning the same topic were included and analyzed in the same way.
2) Selection of studies
The studies were individually selected by at least two experts for each key question and reselected according to the following exclusion criteria. If there was a disagreement between reviewers, the final documents were selected via consensus decision.
3) Exclusion criteria for studies
(1) Suitability of study design: Studies that did not follow the pre-determined research design examining key questions were excluded.
(2) Suitability of subjects: For the literature included in the systematic review, pediatric patients were excluded.
(3) Follow-up period and number of subjects: Randomized trials and systematic reviews were not excluded due to the follow-up period and number of subjects.
(4) Language and publication year: Only the literature published in Korean or English were included. Literature was not excluded on the grounds of the year of publication.
4) Methods to drive recommendations
Primary recommendations were made by at least two subcommittee members. The final recommendation was confirmed after the review and vote of committee. The strength of the recommendation was decided by a majority decision of the committee.
5) Quality of evidence
The quality of evidence for the selected literature was evaluated using two procedures: the quality evaluation of individual studies and assessment of the evidence level for each key question. The quality of individual studies was assessed using the Cochrane's bias assessment tool. The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. In the GRADE methodology, randomized controlled studies were rated as a high level of evidence. The level of evidence was downgraded according to five characteristics: risk of bias, inconsistency, indirectness, imprecision, and publication bias. In the development of this guideline, the assessment outcome is presented in each evidence table by applying this method. Evidence levels were categorized as high, moderate, low, and very low. In addition, if there is no evidence but a recommendation is required, it was created based on an expert opinion. Evidence levels are shown in the following table (
Table 1).
6) Strength of recommendation
The strength of the recommendations was determined by the balance of favorable and unfavorable effects, quality of evidence, preference of patient, and medical costs and resource allocation. In other words, if desirable effects clearly outweigh the undesirable effects, they were strongly recommended, and if desirable effects were closely balanced with undesirable effects or there could be some differences according to individual condition and preference of the patients, they were weakly recommended (
Table 2).
Classification of Idiopathic Interstitial Pneumonia
ILD is a characterized that abnormal collagen accumulation in the lungs due to the proliferation of the interstitial compartment accompanied by infiltration of inflammatory cells and fibrosis. There are many opinions on the classification and range of ILD, but it can be primarily classified with and without known causes. ILD with a known causal etiology can be subdivided into four types according to its cause (
Figure 1). First, environmental ILD includes occupational diseases such as silicosis, asbestosis, berylliosis as well as hypersensitivity pneumonitis (HP). Second, iatrogenic ILD includes ILD caused by radiation or drugs such as chemotherapeutic agents, or anti-arrhythmics. Third, autoimmune ILD includes ILD caused by connective tissue or autoimmune diseases, such as rheumatoid arthritis (RA) and lupus. In addition, lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (PLCH), and pulmonary alveolar proteinosis are also included in the ILD category. ILD without a determinate causative etiology is defined as idiopathic interstitial pneumonia (IIP). There are various types of IIP classified on the basis of histological findings, with prognoses and treatments that are markedly different from each other. For the diagnosis of different types of IIP, radiological, histological, and clinical aspects should be considered. Among the various types of ILDs, this guideline focuses on IIP.
1. Classification of IIP
This classification was established based on the 2013 American Thoracic Society/European Respiratory Society (ATS/ERS) classification
1, which is an update of the 2002 ATS/ERS classification (
Table 3)
2. This classification is based on multidisciplinary diagnosis (MDD), a decision-making process that involves clinicians, radiologists, and pathologists, as well as clinical data that includes smoking history, exposure to hazardous materials (drugs), occupational history, other medical history, and results of pulmonary function tests (PFTs) (
Table 4). Cases with a known cause, such as inhalation of hazardous material, drugs, or CTDs, are not included in IIP.
1) Important differential diagnostic considerations
(1) HP: Chronic HP is sometimes difficult to distinguish from IPF or NSIP despite chest high-resolution computed tomography (HRCT) or lung biopsy. A detailed search for potential exposure to causative agents and specific circulating IgG antibodies may be of helpful, but up to 30% of subjects with histologic HP have no identifiable exposure.
(2) CTD: CTD is a frequent cause of interstitial pneumonia patterns, especially NSIP. Clinical, serologic, HRCT, and histologic findings may be helpful in distinguishing IIPs from ILD associated with CTD. Various forms of ILD occur in RA, systemic lupus erythematosus, systemic sclerosis, and Sjögren's syndrome
3.
(3) Familial interstitial pneumonia: Family history is reported in 2% to 20% of cases of IIP, with heterozygous mutations in
SFTPC,
SFTPA2,
TERT, and
TERC are responsible for about 20% of all familial interstitial pneumonias
4,5. Recently, a promoter variant of the
MUCB gene was reported to be associated with the development of both familial and sporadic IPF
6.
(4) Coexisting pattern: Most patients with a chronic IIP can be given a single clinical-radiologic-pathologic diagnosis. However, multiple pathologic and/or HRCT patterns may be found in the same patient. In smokers, PLCH, respiratory bronchiolitis-ILD (RB-ILD), desquamative interstitial pneumonia, usual interstitial pneumonia (UIP), and emphysema may coexist. Combined pulmonary fibrosis and emphysema is an example of coexisting patterns. Such coexisting patterns may evaluate based on clinical significance of individual patterns through MDD.
2) Rare IIPs
(1) Idiopathic lymphoid interstitial pneumonia: Most are related to autoimmune diseases or lymphoproliferative disorders (lymphoma, post-bone marrow transplant state, human immunodeficiency virus [HIV], Epstein-Barr virus, etc.) and are rarely idiopathic.
(2) Idiopathic pleuroparenchymal fibroelastosis: Pleuroparenchymal fibroelastosis is a rare condition that consists of fibrosis involving the pleura and subpleural lung parenchyma, predominantly in the in the upper lobes. Histologically, the fibrosis is elastotic, and intraalveolar fibrosis is present. Clinically, frequent pneumothorax and recurrent infections are characteristic of this disorder
7.
3) Unclassifiable IIP
The 2002 ATS/ERS classification
2 proposed an “unclassifiable” category of IIP, acknowledging that a final diagnosis may not be achieved despite MDD due to overlapping histological and chest HRCT findings and contradictory clinical, radiological, and pathological findings. This can also occur in CTD and in cases where a biopsy was performed after pharmacological treatment was initiated. A clear classification criteria and data on the clinical presentations of unclassifiable IIP are both not yet well established.
Diagnosis of IIP
1. Medical history
1) Sex
Among different types of ILD, LAM usually occurs in women, particularly in those with child-bearing potential. ILD associated with CTD, with the exception of RA, usually occurs in women as well. On the other hand, pneumoconiosis, PLCH, and IPF occur more frequently in men.
2) Pattern of onset
If the onset of ILD is acute (days to weeks), infection, acute interstitial pneumonia, acute eosinophilic pneumonia, HP, and diffuse alveolar hemorrhage (DAH) should be considered. If the onset is subacute (weeks to months), COP, sarcoidosis, chronic eosinophilic pneumonia (CEP), and drug-induced disease should be considered. If the onset is chronic (months to years), IPF, pneumoconiosis, sarcoidosis, and PLCH should be considered.
3) Occupational history
It is crucial to note not only the current occupation of the patient but also the type and duration of all previous occupations, as well as his or her role and work environment in those.
4) Hobbies and other environmental history
In case of HP, environmental factors such as presence of pets or exposure to animals in the outdoors are important. Clinical pattern of improving symptoms with avoidance of exposure followed by aggravates with re-exposure is useful for the diagnosis of HP.
5) Medication history
Past and current medication histories are both important. Gastric juice aspiration due to gastroesophageal reflux disease slowly developed ILD. Mineral oil, a laxative, and oily nose drops can also induce ILD. Medication use and the order and duration of symptom onset are important; however, ILD may occur weeks to years after the use of relevant medications. History of radiotherapy or high-dose oxygen therapy is also important.
6) Smoking history
Smoking history is very important. More than 90% of PLCH patients have positive smoking history at the time of diagnosis. Patients with RB-ILD or Goodpasture syndrome have been observed to have a prominent history of smoking. In patients who have been exposed to asbestos, interstitial fibrosis occurs 13 times more frequently in smokers compared with non-smokers. Sarcoidosis and HP normally occur in non-smokers.
7) Family history
Family history is important in various genetic metabolic disorders, although they are rare in Korea. Familial incidence can also be seen in sarcoidosis or IIP.
8) Travel and other history
Travel history is important because parasitic infections can cause eosinophilia in the lungs. History of risk factors to HIV is also important.
2. Symptoms
Symptoms of ILD can occur over months to years and can manifest with various levels of progression. Major symptoms include gradually progressing shortness of breath and cough. Wheezing sounds can rarely occur in CEP and HP; substernal chest pain can rarely occur in sarcoidosis. Pleuritic pain can accompany CTD and drug-induced ILD. Chest pain due to pneumothorax may spontaneously occur in PLCH, LAM, tuberous sclerosis, or neurofibromatosis. Hemoptysis typically occurs in DAH, LAM, and pulmonary veno-occlusive disease. Hemoptysis in ILD indicates a high probability of a tumor combine.
3. Physical examination
Crackles are usually heard in the lower lobes of bilateral lungs. Clubbing is observed in progressive fibrotic disease, and pulmonary hypertension or cor pulmonale due to chronic hypoxemia can be seen in its late stages.
4. Radiological findings
1) Chest X-ray
Though chest X-ray is not as sensitive in the diagnosis of ILD as HRCT, it is the first step in identifying ILD. ILD usually presents with reticular pattern, nodular pattern, ground-glass pattern, and consolidation in the bilateral lower lobes on chest X-ray
2. Chest X-ray may appear normal in the earlier stages of ILD.
2) HRCT
Chest HRCT can assess the presence of interstitial pneumonia, the distribution, characteristics, and severity of lung lesions, as well as the presence of combined other lung diseases.
5. Laboratory findings
In patients with suspected ILD, the role of testing for autoimmune antibodies related to CTD is unclear. However, if there are symptoms suggestive of CTD, testing for related autoimmune antibodies is recommended. In a 2011 guideline, screening for rheumatoid factor (RF), anti-cyclic citrullinated peptide, and anti-nuclear antibody (ANA) is recommended even without symptoms suggestive of CTD
8. In a United States study, 22% of IPF patients tested positive for autoimmune antibodies, and these patients showed better prognoses than autoimmune antibody-negative patients. A recent study reports that, among patients who showed UIP pattern, those that tested positive for one or more autoimmune antibodies or possessed one or more symptoms or signs of CTD without a definitive diagnosis of it had better prognoses than IPF patients without such findings.
1) Specific antibody
Positive antibodies against organic dust or proteins confirm a prior exposure but cannot be used singly for the diagnosis of HP. Specific antibodies such as anti-glomerular basement membrane antibody or anti-neutrophil cytoplasmic antibody can be utilized.
2) Non-specific antibody
ANA, RF, and Scl-70 can be helpful in the diagnosis of interstitial pneumonia accompanied by CTD.
3) Angiotensin converting enzyme
Measurement of blood angiotensin converting enzyme concentrations can be helpful in the diagnosis of sarcoidosis.
6. PFT and arterial gas analysis
The characteristic PFT findings are decreased lung compliance, restrictive changes of the lung volumes, particularly forced vital capacity (FVC) and total lung capacity, but normal forced expiratory volume in 1 second/FVC ratios and airway resistance. In most patients, the diffusion capacity of the lungs decreases, and arterial gas analysis during the stable phase can show normal results or hypoxemia and respiratory alkalosis due to ventilation/perfusion (V/Q) mismatch. Hypoxemia characteristically worsens during exercise due to a V/Q mismatch as well as decreased diffusion capacity.
7. Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) is performed by inserting a flexible bronchoscope into a bronchial branch and washing out the cells and materials within the bronchioles and alveoli with 30 to 50 mL of sterile physiological saline solution. In a healthy non-smoker, 90% of the washed-out cells from the alveoli are macrophages, 10% are lymphocytes, and less than 1% are neutrophils. Types of cells that proliferate can vary by disease, and this may be helpful in the differential diagnosis of ILD. Lymphocytes increase in number in cellular NSIP, HP, or COP, which is different from IPF, where neutrophil proliferation is more commonly seen. As these results are non-specific and low in diagnostic value, not all patients need to undergo BAL. However, it can be performed with the discretion of the treating clinician
3.
8. Lung biopsy
Lung biopsy is the most definitive diagnostic tool and includes transbronchial lung biopsy (TBLB), transbronchial lung cryobiopsy (TBLC), and surgical lung biopsy (SLB) (via open thoracotomy and video-assisted thoracoscopic biopsy).
1) TBLB
Conditions commonly diagnosed with TBLB include lung sarcoidosis, malignant tumors (bronchoalveolar carcinoma), lymphangitic carcinomatosis, alveolar proteinosis, infections such as Pneumocystis jirovecii pneumonia or tuberculosis, and eosinophilic pneumonia.
2) TBLC
Surgical biopsy is known to be the standard histological investigation. However, surgical biopsy is disadvantageous with regard to cost and risk. A recent study reported that TBLB performed using a cryoprobe can harvest a 40 to 50 mm
2 lung tissue sample and that its diagnostic contribution in patients with high suspicion of IIP is as high as surgical biopsy
9. By TBLC, sufficient lung tissue can be obtained, and definitive histological diagnosis can be made by a pathologist. The concurrence rate of histological diagnosis among pathologists in the diagnosis of UIP is also high. Pneumothorax as a complication is reported in as high as 28% of cases, but the number of studies conducted with standard methods is still low. Therefore, further research is needed.
3) Video-assisted thoracoscopic surgery
SLB is the most useful diagnostic tool in ILDs but must be performed selectively with consideration for patient age, systemic status, comorbidities, and complications
8,10. Indications include progressive lesions with inconclusive chest HRCT, predictable drug reactions to therapies high in rates of adverse events, such as immunosuppressant use, or the necessity to distinguish the progression of IIP from cancer or infection
10,11. Relative contraindications include diffuse end-stage lung disease (honeycomb lesion) due to the high probability of obtaining only fibrotic lung tissue, accompanying severe emphysema, a less than 35% predictive value of lung diffusion capacity, severe hypoxia, and severe heart disease
10,11. The area of biopsy that can best represent the overall lesion is determined using chest HRCT; while avoiding late-stage honeycomb lesions, two or more tissue samples of sufficient sizes are obtained from different lobes. The right middle lobe or lingular segment of the left upper lobe frequently develops non-specific inflammation and passive congestion and is best avoided. Though biopsy is important for diagnosis, it alone cannot be used for definitive diagnosis of ILD and must be accompanied by clinical and radiological findings.
9. Biomarkers
Researchers so far have been greatly interested in the discovery of biomarkers for IIP. As a result, a few interesting results on the diagnosis, treatment, and prognosis of ILD have been observed (
Table 5). For example, high serum levels of proteins related to epithelial cells or macrophages, such as surfactant protein (SP)-A, SP-D, Krebs von den Lungen-6, chemokine ligand (CCL)-18, and matrix metalloproteinase-7, were found to be associated with rapid decline in pulmonary function and decreased survival rate. These proteins can be used as clinically useful biomarkers to identify patients at high risk of disease progression. Serum SP-A is significantly higher in IPF than in NSIP or COP, while SP-D is significantly higher in CTD-associated ILD than in IPF.
BAL fluid in NSIP shows a helper T-cell type 1-like pattern. In contrast, IPF has an increased helper T-cell type 2-like response and increased expression of chemokine receptor-7 and CCL7.
Table 5 represents the biomarkers within the blood and BAL fluid that are related to survival
1.
Figure 1
Classification of interstitial lung disease (ILD). ILD can be primarily classified with and without known causes. Known etiologies of ILD were occupational or environmental exposures, drugs, radiation, connective tissue diseases and so on.
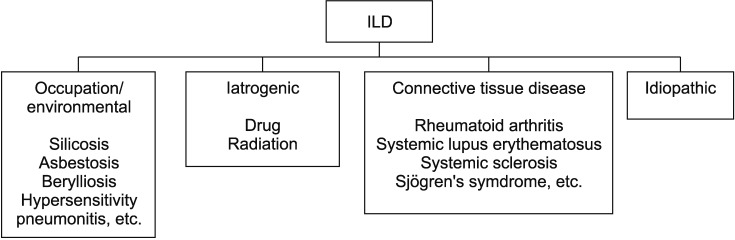
Table 1
Definition of quality of evidence
|
The quality of evidence |
Definition |
|
High |
Further research is very unlikely to change our confidence in the estimate of the effect. |
|
Moderate |
Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
|
Low |
Further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate. |
|
Very low |
Any estimate of effect is very uncertain. |
|
Expert opinion |
Expert opinions, based on clinical experience, without any scientific evidence. |
Table 2
Definition of strength of recommendation
|
Strength of recommendation |
Definition |
|
Strong recommendation |
All or almost all individuals should receive the recommended course of action. |
|
Weak recommendation |
The majority of individuals should receive the suggested course of action, but many would not. The best action may differ depending on circumstance or preference. |
Table 3
Revised American Thoracic Society/European Respiratory Society classification of idiopathic interstitial pneumonias: multidisciplinary diagnoses
|
Major idiopathic interstitial pneumonias |
|
Idiopathic pulmonary fibrosis |
|
Idiopathic non-specific interstitial pneumonia |
|
Respiratory bronchiolitis-interstitial lung disease |
|
Desquamative interstitial pneumonia |
|
Cryptogenic organizing pneumonia |
|
Acute interstitial pneumonia |
|
Rare idiopathic interstitial pneumonias |
|
Idiopathic lymphoid interstitial pneumonia |
|
Unclassifiable idiopathic interstitial pneumonias |
Table 4
Categorization of idiopathic interstitial pneumonias by duration of symptoms and smoking history
|
Category |
Clinical-radiologic-pathologic diagnoses |
Associated radiologic and/or pathologic-morphologic patterns |
|
Chronic fibrosing interstitial pneumonia |
Idiopathic pulmonary fibrosis |
Usual interstitial pneumonia |
|
Idiopathic non-specific interstitial pneumonia |
Non-specific interstitial pneumonia |
|
Smoking-related interstitial pneumonia |
Respiratory bronchiolitis-interstitial lung disease |
Respiratory bronchiolitis |
|
Desquamative interstitial pneumonia |
Desquamative interstitial pneumonia |
|
Acute/subacute interstitial pneumonia |
Cryptogenic organizing pneumonia |
Organizing pneumonia |
|
Acute interstitial pneumonia |
Diffuse alveolar damage |
Table 5
Biomarkers for outcome in blood and bronchoalveolar lavage (higher levels predicting poor survival)
|
Biomarker |
Patient |
HR (95% CI) |
p-value |
|
SP-A |
52 IPF (survivors vs. non-survivors) |
- |
0.013 |
|
SP-D |
- |
0.003 |
|
SP-A |
142 IPF |
1.73 |
0.031 |
|
SP-D |
- |
2.04 |
0.003 |
|
KL-6 (>1,000 U/mL) |
27 IPF |
12.56 (1.20-131.90) |
0.035 |
|
KL-6 (≥1,000 U/mL) |
152 IIP and 67 CVD |
2.95 (1.71-5.08) |
0.000 |
|
SP-D (≥253) |
82 IPF |
- |
0.001 |
|
SP-A |
- |
- |
NS |
|
KL-6 (>1.014) |
- |
- |
0.009 |
|
Oxidative stress levels |
21 IPF |
FVC (r=−0.79) |
<0.010 |
|
DLco (r=−0.75) |
<0.010 |
|
MMP-7 |
74 IPF |
Higher decline of DLco (r=−0.53) and FVC (r=−0.51) |
0.002 |
|
MMP-1 |
- |
0.002 |
|
SP-A |
82 IPF |
3.27 (1.49-7.17) |
0.003 |
|
SP-D (>460 ng/mL) |
72 IPF |
3.22 (1.33-7.81) |
0.010 |
|
CCL18 (>150 ng/mL) |
72 IPF |
7.98 (2.49-25.51) |
0.001 |
|
CD4+CD28null >18% of total CD4 |
89 IPF |
13.00 (1.60-111.10) |
0.000 |
|
MMP-7, ICAM-1, IL-8, VCAM-1, S100-A12 |
241 IPF derivation; 101, validation |
In the derivation cohort, high concentration predicted poor survival, poor transplant-free survival and poor progression-free survival |
Overall survival derivation cohort |
|
In the validation cohort, high concentrations of all five were predictive of poor transplant-free survival; MMP-7, ICAM-1, and IL-8 of overall survival; and ICAM-1 of poor progression-free survival |
MMP-7: 0.002 |
|
ICAM-1: 0.002 |
|
IL-8: 0.029 |
|
VCAM-1: 0.000 |
|
S100-A12: 0.001 |
|
BAL |
20 IPF |
Higher in rapid progressors |
0.028 |
|
MMP-8, MMP-9 |
- |
- |
0.015 |
|
BAL |
39 IPF |
Higher in non-survivors |
<0.020 |
|
CCL2 |
- |
- |
- |
|
BAL |
20 IPF |
Negative correlation with PFT |
- |
|
Endostatin |
- |
FVC (r=−0.604) |
0.006 |
|
- |
TLco (r=−0.612) |
0.005 |







 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation