 |
 |
| Tuberc Respir Dis > Volume 84(1); 2021 > Article |
|
Abstract
Notes
Authors’ Contributions
Conceptualization: Jirjees FJ. Methodology: Jirjees FJ, Dallal Bashi YH. Formal analysis: Dallal Bashi YH. Data curation: Dallal Bashi YH, Jirjees FJ, Al-Obaidi HJ. Software: Dallal Bashi YH. Validation: Dallal Bashi YH, Jirjees FJ. Investigation: Jirjees FJ, Dallal Bashi YH, Al-Obaidi HJ. Project administration: Jirjees FJ. Supervision: Jirjees FJ. Visualization: Dallal Bashi YH, Al-Obaidi HJ. Writing - original draft preparation: Jirjees FJ, Dallal Bashi YH. Writing - review and editing: Jirjees FJ, Dallal Bashi YH, Al-Obaidi HJ. Approval of final manuscript: all authors.
Fig. 1.
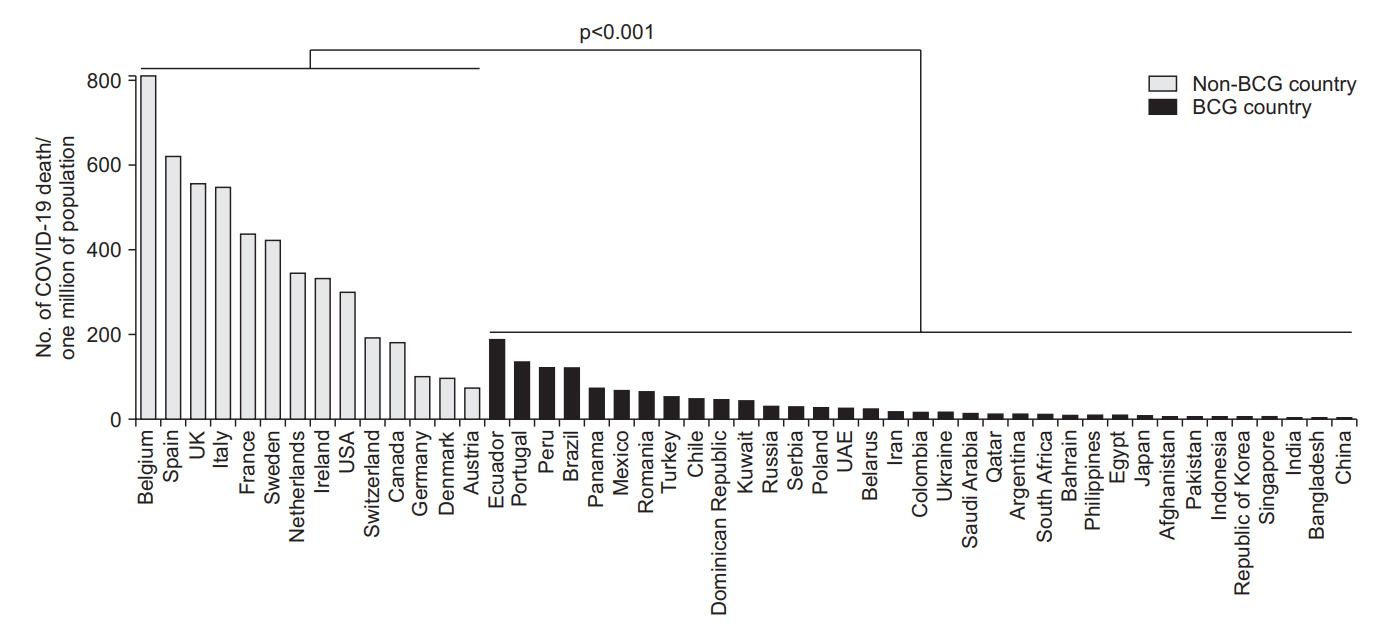
Table 1.
| No. | Country | Total COVID-19 cases | Total COVID-19 death cases | Case per 1 million | Death per 1 million |
|---|---|---|---|---|---|
| Non-BCG countries | |||||
| 1 | Belgium | 57,849 | 9,388 | 4,991 | 810 |
| 2 | Spain | 238,278 | 29,037 | 5,096 | 621 |
| 3 | UK | 269,131 | 37,837 | 3,964 | 557 |
| 4 | Italy | 231,732 | 33,142 | 3,833 | 548 |
| 5 | France* | 146,122 | 28,608 | 2,239 | 438 |
| 6 | Sweden† | 35,727 | 4,266 | 3,538 | 422 |
| 7 | Netherlands | 45,950 | 5,903 | 2,682 | 345 |
| 8 | Ireland‡ | 24,841 | 1,639 | 5,031 | 332 |
| 9 | USA | 1,675,258 | 98,889 | 5,061 | 299 |
| 10 | Switzerland | 30,713 | 1,654 | 3,549 | 191 |
| 11 | Canada | 87,902 | 6,799 | 2,329 | 180 |
| 12 | Germany | 180,458 | 8,450 | 2,154 | 101 |
| 13 | Denmark | 11,512 | 568 | 1,987 | 98 |
| 14 | Austria | 16,543 | 668 | 1,837 | 74 |
| Median | 72,876 | 7,625 | 3,543 | 338 | |
| BCG countries | |||||
| 15 | Ecuador | 38,471 | 3,313 | 2,181 | 188 |
| 16 | Portugal§ | 31,596 | 1,369 | 3,099 | 134 |
| 17 | Peru | 135,905 | 3,983 | 4,122 | 121 |
| 18 | Brazil | 411,821 | 25,598 | 1,937 | 120 |
| 19 | Panama | 11,728 | 315 | 2,718 | 73 |
| 20 | Mexico | 78,023 | 8,597 | 605 | 67 |
| 21 | Romania | 18,791 | 1,229 | 977 | 64 |
| 22 | Turkey | 160,979 | 4,461 | 1,909 | 53 |
| 23 | Chile | 86,943 | 890 | 4,548 | 47 |
| 24 | Dominican Republic | 16,068 | 485 | 1,481 | 45 |
| 25 | Kuwait | 24,112 | 185 | 5,646 | 43 |
| 26 | Russia | 387,623 | 4,374 | 2,656 | 30 |
| 27 | Serbia | 11,300 | 241 | 1,293 | 28 |
| 28 | Poland | 22,825 | 1,038 | 603 | 27 |
| 29 | UAE | 32,532 | 258 | 3,289 | 26 |
| 30 | Belarus | 39,858 | 219 | 4,218 | 23 |
| 31 | Iran | 143,849 | 7,627 | 376 | 16 |
| 32 | Colombia | 24,104 | 803 | 474 | 16 |
| 33 | Ukraine | 22,811 | 679 | 522 | 16 |
| 34 | Saudi Arabia | 80,185 | 441 | 2,303 | 13 |
| 35 | Qatar | 50,914 | 33 | 17,672 | 11 |
| 36 | Argentina | 13,933 | 501 | 308 | 11 |
| 37 | South Africa | 27,403 | 577 | 462 | 10 |
| 38 | Bahrain | 10,052 | 15 | 5,907 | 9 |
| 39 | Philippines | 15,588 | 921 | 142 | 8 |
| 40 | Egypt | 20,793 | 845 | 203 | 8 |
| 41 | Japan | 16,719 | 874 | 132 | 7 |
| 42 | Afghanistan | 13,659 | 246 | 351 | 6 |
| 43 | Pakistan | 64,028 | 1,317 | 290 | 6 |
| 44 | Indonesia | 24,538 | 1,496 | 90 | 5 |
| 45 | Republic of Korea | 11,402 | 269 | 222 | 5 |
| 46 | Singapore | 33,249 | 23 | 5,683 | 4 |
| 47 | India | 165,799 | 4,706 | 120 | 3 |
| 48 | Bangladesh | 40,321 | 559 | 245 | 3 |
| 49 | China | 84,547 | 4,645 | 59 | 3 |
| Median | 31,596 | 845 | 977 | 16 |
Table 2.
References
-
METRICS

- ORCID iDs
-
Feras J. Jirjees

https://orcid.org/0000-0001-6265-0808Yahya H. Dallal Bashi

https://orcid.org/0000-0002-8480-9776 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



