|
 |
| Tuberc Respir Dis > Volume 82(1); 2019 > Article |
|
Abstract
The pathogen Mycobacterium avium complex (MAC) is the most common cause of nontuberculous mycobacterial pulmonary disease worldwide. The decision to initiate long-term antibiotic treatment is difficult for the physician due to inconsistent disease progression and adverse effects associated with the antibiotic treatment. The prognostic factors for the progression of MAC pulmonary disease are low body mass index, poor nutritional status, presence of cavitary lesion(s), extensive disease, and a positive acid-fast bacilli smear. A regimen consisting of macrolides (clarithromycin or azithromycin) with rifampin and ethambutol has been recommended; this regimen significantly improves the treatment of MAC pulmonary disease and should be maintained for at least 12 months after negative sputum culture conversion. However, the rates of default and disease recurrence after treatment completion are still high. Moreover, treatment failure or macrolide resistance can occur, although in some refractory cases, surgical lung resection can improve treatment outcomes. However, surgical resection should be carefully performed in a well-equipped center and be based on a rigorous risk-benefit analysis in a multidisciplinary setting. New therapies, including clofazimine, inhaled amikacin, and bedaquiline, have shown promising results for the treatment of MAC pulmonary disease, especially in patients with treatment failure or macrolide-resistant MAC pulmonary disease. However, further evidence of the efficacy and safety of these new treatment regimens is needed. Also, a new consensus is needed for treatment outcome definitions as widespread use of these definitions could increase the quality of evidence for the treatment of MAC pulmonary disease.
Nontuberculous mycobacteria (NTM) are ubiquitous organisms that can be isolated from the environment, including water and soil1, and they cause pulmonary and extrapulmonary disease2,3. Pulmonary disease (PD) is the most common clinical presentation of NTM infection, and although the cause of NTM-PD development is not clearly understood, it may be associated with both host and bacterial factors4,5,6,7. Mycobacterium avium complex (MAC) is the most common cause of NTM-PD worldwide4,5,6,7.
The number of recognized NTM organisms is increasing, with more than 180 species currently recognized (http://www.bacterio.net/mycobacterium.html). The distribution of NTM species varies widely according to geography8. The number of MAC organisms is also increasing and currently includes M. avium, M. intracellulare, M. chimaera, M. arosiense, M. bouchedurhonense, M. colombiense, M. marseillense, M. timonense, M. vulneris, and M. yongonense4. Within the complex, M. avium, M. intracellulare, and M. chimaera are the most common human pathogens4. The distribution of each MAC organism varies according to country; for example, M. chimaera is relatively rare in South Korea9,10 but common in Germany11. Notably, M. chimaera is less virulent than M. avium or M. intracellulare11,12, and the recurrence rates of MAC-PD after successful treatment completion also may differ for MAC species12.
MAC-PD has two different clinical phenotypes: the fibrocavitary and nodular bronchiectatic forms4,5,6,7. The fibrocavitary form is typified by a cavitary lesion in the upper lobes and is usually associated with other pulmonary diseases. For example, the lesions can stem from scars from previous pulmonary tuberculosis and/or chronic obstructive pulmonary disease, which frequently develops in older men and shows rapid progression2. The nodular bronchiectatic form is characterized by bilateral bronchiectasis with multiple nodules and tree-inbud opacities often in the right middle lobe and the lingular segment of the left upper lobe on high-resolution computed tomography (HRCT)2. This form usually occurs in postmenopausal, non-smoking females and has a slow progression2. Additionally, cavitary lesions can also be associated with the nodular bronchiectatic forms13,14,15,16,17.
The incidence and prevalence of NTM-PD are increasing worldwide, affecting both immunocompetent and immunocompromised individuals18,19. In South Korea, the frequency of NTM isolation from clinical specimens and the number of patients with NTM-PD have been increasing over the past decade7, which may be mainly due to the increasing incidence of MAC-PD20. Although M. intracellulare was more prevalent than M. avium among MAC-PD cases in the past, M. avium has recently become more frequently isolated than M. intracellulare in South Korea20,21,22,23,24. Despite the increasing prevalence of MAC-PD, its treatment remains difficult. In this report, we review the treatment of MAC-PD.
Diagnosis of MAC-PD does not require immediate initiation of treatment2,3, and understanding the potential for progression is very important. A significant proportion of patients with MAC-PD (approximately 40%-60%) remain without disease progression for several years after diagnosis, even without treatment12,13,15,25,26. Moreover, approximately 40%-50% of patients with untreated MAC-PD achieve spontaneous negative culture conversion without antibiotic treatment26,27,28. Therefore, to avoid unnecessary treatments that might cause unwarranted medical expenses and adverse drug reactions, clinicians should consider the risk of disease progression and make timely decisions in the treatment initiation phase (Figure 1).
Important prognostic factors related to the progression of MAC-PD are low body mass index and poor nutritional status13,26,27,29,30, the presence of cavitary lesions13,15,25,26,30, extensive disease26,29, and positive acid-fast bacilli (AFB) smears25,26,27, although this evidence has been supported by case series rather than by randomized, controlled studies.
Patients with MAC-PD have different treatment prognoses based on the severity of an individual patient's disease, previous treatment history, and resistance of the strain to various drugs2,3. The American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines published in 2007 recommend that a macrolide-based, three-drug combination regimen, consisting of macrolides (clarithromycin or azithromycin) with rifampin and ethambutol, should be administered for at least 12 months after sputum cultures convert to negative2. This regimen can be strengthened by adding parenteral drugs, such as streptomycin or amikacin, for patients with severe and extensive, especially fibrocavitary disease. The frequency of administration of the treatment regimen is also different: three times per week administration is recommended for patients with non-cavitary nodular bronchiectatic disease; and a daily treatment is recommended for cavitary MAC-PD (Figures 2, 3, 4). Intermittent therapy has potential benefits for decreasing adverse drug effects and medication costs and for increasing adherence to medication31,32. The recent British Thoracic Society (BTS) guidelines, published in 2017, basically recommend the same treatment regimen, consisting of a macrolide-based, three-drug combination regimen for MAC-PD3. The BTS guidelines also recommend two different treatment regimens, i.e., intermittent therapy for non-severe MAC-PD and daily therapy for severe MAC-PD. However, the indications differ slightly from those of the previous ATS/IDSA guidelines. The BTS guidelines recommend against using an intermittent oral antibiotic regimen in patients with severe MAC-PD, such as in cases with AFB smear-positive respiratory tract samples, radiologic evidence of lung cavitation/severe infection, or severe symptoms/signs of systemic illness3, and therefore do not limit daily treatment to only cavitary disease. These two sets of guidelines, which suggest the same regimens for treating MAC-PD, were primarily based on the outcomes of observational cohort studies, case series, and expert opinions due to a paucity of randomized, controlled trials for this disease4. However, adherence to the recommended regimens of the current guidelines has been found to be poor33,34.
Since 2004, five systematic reviews and meta-analyses have been published on treatment outcomes for MAC-PD (Table 1)35,36,37,38,39. The treatment success rates in individual studies varied because of their significant heterogeneity, including different definitions of treatment outcomes, inclusion and exclusion criteria, disease severities, regimens, and dosages. The pooled treatment success rates in the five systematic reviews ranged from 32% to 65%, and 12%-16% of the enrolled patients had not completed treatment35,36,37,38,39. In a subgroup analysis of these systematic reviews, macrolide-containing regimens showed better outcomes compared to those without macrolides35,36,37,38,39. Furthermore, use of the triple-drug regimens recommended by the ATS/IDSA was also associated with better outcomes, and the success rates of these regimens further increased for macrolide-susceptible patients and those with previously untreated MAC-PD when the drugs were taken for at least one year39.
Although the pooled success rate of treatment in the meta-analysis was relatively low, culture conversion rates were relatively high in recent large cohort studies among patients with MAC-PD who received more than 12 months of the recommended three-drug therapy (74%-88%)17,31,32,40. Among these large cohort studies, one recent study of 481 patients with MAC-PD in South Korea showed that treatment outcomes differed according to clinical phenotypes17. A favorable outcome, defined as sputum culture conversion after initiation of treatment and maintenance of negative culture for at least 12 months of treatment, was higher in patients with non-cavitary nodular bronchiectatic disease (88%) compared to those with cavitary nodular bronchiectatic (78%) and fibrocavitary disease (76%) (p<0.05), regardless of the use of daily treatment and streptomycin with the latter two phenotypes17. However, it should be noted that this study did not use an intention-to-treat analysis and excluded 85 patients who did not complete at least 12 months of treatment due to drug intolerance, follow-up loss, death, and lack of perceived benefit; if these patients had been included in the denominator, the overall success rate would be reduced from 84% to 71%41.
Regarding the frequency of treatment, intermittent therapy given three times weekly shows comparable high treatment success rates with high tolerability and requires fewer regimen modifications when compared to daily therapy31,32. Adverse effects such as visual disturbance or optic neuropathy due to ethambutol are less likely to be observed in intermittent therapy than in daily therapy32,42. Therefore, this intermittent treatment should be considered in patients with non-cavitary nodular bronchiectatic disease, in contrast to patients with cavitary MAC-PD in which intermittent therapy results in low culture conversion rates and low HRCT improvement43. Although the usually recommended dose of azithromycin is 250 mg2,3, sometimes a 500 mg daily dose of azithromycin is also recommended especially for cavitary MAC-PD44,45 (Table 2).
Nevertheless, overall, the treatment success rate with the combination antibiotic regimen has been unsatisfactory, and a substantial proportion of patients remain culture-positive with refractory MAC-PD despite long-term antibiotic therapy36,37,38,39. In patients with refractory MAC-PD who undergo long-term macrolide therapy, however, macrolide resistance develops infrequently31,32. A recent study showed that refractory MAC-PD was commonly caused by frequent reinfection with new MAC strains during antibiotic treatment rather than by persistence of the original strains, which could help to explain the infrequent development of macrolide resistance in patients with refractory MAC-PD46.
Although microbiologic recurrences of MAC-PD, even after successful completion of antibiotic therapy, are relatively common, optimum treatment approaches for recurrent disease have not been determined. Treatment outcomes in patients with a history of previous treatment of MAC-PD tend to be worse than the outcomes in newly treated patients47,48,49,50, and therefore a strengthened regimen that includes a daily macrolide-containing three drug regimen combined with parenteral amikacin or streptomycin is recommended for these patients with recurrent MAC-PD2. However, evidence suggests that the majority of MAC recurrences after therapy completion are reinfections rather than relapses or treatment failures17,31, and so these strengthened regimens might be excessive and cause unnecessary adverse drug reactions. A recent cohort study showed that a three-times-weekly intermittent therapy in patients with previously treated non-cavitary nodular bronchiectatic MAC-PD achieved a high sputum culture conversion rate that was comparable with that of daily therapy (82% vs. 81%, p=0.999)51. Therefore, intermittent treatment could be considered in patients with recurrent non-cavitary nodular bronchiectatic MAC-PD when the isolate remains susceptible to macrolides.
The treatment of patients with macrolide-resistant MAC-PD is challenging because of very poor treatment outcomes and lack of effective drugs52,53,54,55. For macrolide-resistant MAC-PD, culture conversion rates of only 15%-36% have been reported as well as 2-year and 5-year mortalities of 9%-15% and 47%53,54, respectively. Risk factors for the development of macrolide resistance are macrolide monotherapy, the use of macrolide plus rifampin without ethambutol, and the use of macrolide in combination with fluoroquinolone only52,53,54,55. Therefore, these treatment regimens should be avoided for the treatment of MAC-PD, especially in patients with high bacterial burden such as in cavitary disease. However, macrolide-resistant MAC can still develop in some patients treated with guideline-based therapy53,54. In cases where sputum is persistently AFB smear-positive during treatment, even when guideline-based treatment is used, the patient should be carefully monitored for the development of macrolide resistance.
The recently published BTS guidelines recommend adding additional drugs such as isoniazid or moxifloxacin and intravenous or nebulized amikacin to the standard antibiotic treatment for macrolide-resistant disease, although the efficacy of these treatments remains inconclusive3. One study looked at 41 patients with refractory MAC-PD that was defined as a persistent AFB smear-positive sputum culture for at least six months after initiation of a macrolide-based standard regimen; the addition of moxifloxacin was associated with culture conversion in 33% of the patients with macrolide susceptible isolates but none of the patients with macrolide resistant isolates56.
Because the success rate of medical therapy alone for MAC-PD has not been suboptimal, and many patients experience treatment failure or macrolide resistance that cannot be successfully treated with medical therapy alone, adjuvant lung resection surgery should be considered in select patients to improve treatment outcomes2,3. The culture conversion rate following surgical treatment has been as high as 80%-100%, despite the fact that most studies included patients who experienced treatment failure57,58. Reported postoperative mortality and morbidity have varied considerably, ranging from 0% to 23% and 0% to 50%, respectively57,58. Mortality and morbidity were higher in those who underwent pneumonectomy compared to those who had other operative procedures57,58. However, mortality and morbidity decreased from the early to the late phase of the study period in a large cohort study conducted at a single, experienced center59; this improvement was likely associated with improved patient selection, better preoperative assessments including nutritional assessment and antibiotic therapy as a part of a multidisciplinary treatment approach, surgical techniques, and postoperative measures.
Surgical resection is most commonly indicated due to treatment failures during medical therapy; however, other indications also include hemoptysis, cavitary disease, destructive lung lesions, and lung nodules that are limited in site and extent. Because treatment failure can be expected in patients with a positive culture despite 6-12 months of therapy, surgical therapy should be considered in these patients. Considering the recommended treatment duration of 12 months after sputum culture conversion to negative, postoperative antibiotic treatment should also be administered for 12 months if sputum culture conversion is achieved after surgical resection60.
Studies on surgical resection for NTM-PD have been retrospective and performed in several experienced centers. Therefore, there could be a selection bias, and the treatment outcomes, such as treatment success and complication rates of surgical resection, might not be generalizable. Because of the significant postoperative morbidity and mortality involved, guidelines recommend that the performance of lung resection surgery be based on a rigorous risk-benefit analysis in a multidisciplinary setting, in an experienced center2,3.
Although macrolide-containing regimens have improved treatment outcomes for MAC-PD, multiple drugs and a long duration of treatment can cause multiple adverse drug reactions, and therefore, the treatment success rate of this regimen is still unsatisfactory. Moreover, macrolide-resistant MAC-PD is usually refractory to current medical treatments. Even after successful treatment, a significant number of recurrences occur, contributing to the suboptimal treatment success rate for MAC-PD. Therefore, novel therapeutic agents are needed to improve treatment outcomes.
The drug clofazimine was developed for tuberculosis in the 1950s but was ultimately used to treat leprosy. Recently, this drug has been used for the treatment of multidrug-resistant tuberculosis, with good efficacy61. Its efficacy for MAC-PD was shown in two cohort studies from a single institute in Canada, in which regimens consisting of a macrolide, ethambutol, and clofazimine were utilized, and 87% (26/30) and 99% (92/93) of patients achieved culture conversion62,63. Adverse drug reactions related to clofazimine in these two studies included skin color changes (Ōēź5%). In a recent large cohort study of 112 patients with MAC and/or M. abscessus infections, most of whom had refractory disease and some of whom also had cystic fibrosis, clofazimine was included in the regimen, and the culture conversion rate for patients with MAC-PD was 42% (11/26)64. In this study, skin-related adverse drug reactions, including skin discoloration, dryness, or sun hypersensitivity, were the most common (66%), followed by gastrointestinal (55%) and neurologic (42%) adverse drug reactions; clofazimine was discontinued in 14% of patients due to the adverse drug reactions64. Although clofazimine can have side effects, given its efficacy, its use should be considered when the guideline-recommended regimen is not administered due to adverse drug reactions, drug-drug interactions, or drug resistance.
Injectable aminoglycosides, including amikacin and streptomycin, have been recommended for MAC-PD if the disease is associated with cavitary disease or macrolide resistance2,3. However, serious systemic side effects, including auditory, vestibular, and renal toxicity could limit the long-term systemic administration of these drugs. Administering amikacin by inhalation may be an effective alternative for increasing the efficacy of the drug and decreasing adverse drug reactions. Although recent BTS guidelines recommended this treatment for severe or macrolide-resistant MAC-PD, reports on this approach are limited. Case series using different doses and frequencies of administration of inhaled amikacin in refractory cases with MAC or M. abscessus infections showed variable culture conversion rates (25%-43%) and adverse drug reaction rates (8%-35%)65,66. Recently, in a large cohort study from South Korea involving 77 patients with refractory NTM-PD (M. abscessus, n=48; MAC, n=20; mixed infection, n=9), all of whom received amikacin inhalation therapy, culture conversion was achieved in 18% of the cases overall and 15% of the MAC-PD cases67. In the same study, adverse drug reactions such as ototoxicity to the amikacin inhalation therapy developed in 38% of all patients, and this therapy was discontinued in 27% of patients67. Although these studies showed clinical and microbiologic improvements in some patients, the treatment was associated with common adverse reactions. In order for inhaled amikacin to be used more widely, optimal dosing and frequency of administration will need to be determined to reduce adverse reactions and improve efficacy.
Previous studies used an injectable formulation of amikacin; however, recently a novel amikacin formulation was developed, i.e., liposomal amikacin for inhalation68. Liposomal amikacin comprises a small, charge-neutral, highly biocompatible liposome with encapsulated amikacin, a formulation that can enhance drug delivery and may lead to improved efficacy and decreased toxicity68. A phase II, double-blind, randomized, placebo-controlled trial for this drug in patients with refractory NTM-PD, including both MAC and M. abscessus, was recently published69. In this study, the treatment group showed a significantly higher proportion of patients with negative cultures at 84 days compared to that of the placebo group (32% vs. 9%, p=0.006)69. However, adverse drug reactions commonly developed in the treatment group, such as dysphonia, bronchiectasis exacerbation, cough, oropharyngeal pain, fatigue, chest discomfort, wheezing, and infective pulmonary exacerbation of cystic fibrosis69. A phase III, randomized, open-label study on refractory MAC-PD, recently released as a conference abstract, showed a higher proportion of negative cultures at six months in patients treated with liposomal amikacin inhalation therapy in addition to guideline-based therapy compared to patients treated with guideline-based therapy only (29% vs. 9%, p<0.001)70. However, respiratory adverse drug reactions were more common in patients taking the inhalation therapy when compared to the control patients (87% vs. 50%), although there were no safety issues for this drug related to nephrotoxicity or ototoxicity.
Bedaquiline is a novel diarylquinoline targeting the proton pump of adenosine triphosphate synthase in M. tuberculosis71,72. The drug was developed to treat multidrug-resistant tuberculosis and has good efficacy and safety when used in combination with the World Health Organization's recommended background regimen for multidrug-resistant tuberculosis. Bedaquiline has also shown potent antimycobacterial activity against MAC, with a low minimal inhibitory concentration in clinical isolates73,74. Only one case series has been published for NTM-PD, which included 10 patients (six with MAC and four with M. abscessus) with refractory NTM-PD and which showed a microbiologic response in 60% of patients and one or more negative cultures in 50% of patients75. Further large studies are needed to confirm the efficacy and safety of bedaquiline for the treatment of refractory or macrolide-resistant MAC-PD.
In contrast to tuberculosis, NTM-PD has no widely accepted treatment outcome definitions and this lack of consensus may decrease the quality and interpretability of results from clinical trials and retrospective cohort studies. Recently, a consensus statement76, proposed definitions for culture conversion, microbiological cure, clinical cure, treatment failure, recurrence, relapse, reinfection, death, and unknown outcomes (Table 3). These uniform definitions could improve the quality of evidence for NTM-PD treatment.
Although the current treatment outcome definitions were primarily based on the results of negative sputum culture conversion rate or cure and mortality, patient-centered approach outcome measurement was also important in patients management and clinical studies77. Patients with NTM-PD have significantly impaired health-related quality of life (HRQL) that is closed associated lung function78,79,80,81,82,83. Therefore, improvement of HRQL may be a reasonable treatment goal for patients with this chronic and difficult-to-treat NTM-PD84. More studies are needed for clarifying the standard assessment for HRQL in patients with NTM-PD which can guide appropriate therapeutic strategies in NTM-PD.
The incidence and prevalence of NTM-PD have been rapidly increasing worldwide, with MAC the most common pathogen. Proper and timely treatment should be considered when patients have any of the prognostic factors for disease progression: a low body mass index, poor nutritional status, cavitary lesion(s), extensive disease, or positive AFB smear. When initiating treatment of MAC-PD, a guideline-recommended, macrolide-based combination therapy using three oral drugs should be started and maintained for at least 12 months after sputum culture conversion to negative. MAC-PD with treatment failure or macrolide resistance is usually refractory to medical treatment alone. Surgical treatment can increase the number of successful treatment outcomes in these patients with localized disease. However, as the complication rates of surgical treatment can be high, surgical resection should be considered following a rigorous risk-benefit analysis in a multidisciplinary setting and should be performed in an experienced center. New and repurposed drugs including clofazimine, inhaled amikacin, and bedaquiline have shown promising results, and further studies of their efficacy and safety are needed. Recently, consensus definitions on culture conversion, microbiological and clinical cure, treatment failure, recurrence, relapse, reinfection, death, and unknown outcomes of NTM-PD were developed and should be used to increase the quality of evidence for treating NTM-PD.
Notes
Conflicts of Interest: Dr. Won-Jung Koh has received a consultation fee from Insmed Inc. for the Insmed Advisory Board Meeting, not associated with the submitted work. Dr. Charles L. Daley has received grants from Insmed, Inc. and served on Advisory Boards for Insmed Inc., Johnson and Johnson, Spero, and Horizon, not associated with the submitted work. Otherwise, we have no conflicts of interest to declare.
References
1. Falkinham JO 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 2015;36:35-41. PMID: 25676517.


2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. PMID: 17277290.


3. Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72:ii1-ii64.

6. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis 2016;79:74-84.

7. Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci 2016;31:649-659. PMID: 27134484.



8. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013;42:1604-1613. PMID: 23598956.


9. Moon SM, Kim SY, Jhun BW, Lee H, Park HY, Jeon K, et al. Clinical characteristics and treatment outcomes of pulmonary disease caused by Mycobacterium chimaera. Diagn Microbiol Infect Dis 2016;86:382-384. PMID: 27720208.


10. Kim SY, Shin SH, Moon SM, Yang B, Kim H, Kwon OJ, et al. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn Microbiol Infect Dis 2017;88:125-137. PMID: 28291631.


11. Schweickert B, Goldenberg O, Richter E, Gobel UB, Petrich A, Buchholz P, et al. Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerg Infect Dis 2008;14:1443-1446. PMID: 18760016.



12. Boyle DP, Zembower TR, Reddy S, Qi C. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species. Am J Respir Crit Care Med 2015;191:1310-1317. PMID: 25835090.


13. Kitada S, Uenami T, Yoshimura K, Tateishi Y, Miki K, Miki M, et al. Long-term radiographic outcome of nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Int J Tuberc Lung Dis 2012;16:660-664. PMID: 22410245.


14. Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;185:575-583. PMID: 22199005.


15. Lee G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, et al. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease: natural course on serial computed tomographic scans. Ann Am Thorac Soc 2013;10:299-306. PMID: 23952847.


16. Gochi M, Takayanagi N, Kanauchi T, Ishiguro T, Yanagisawa T, Sugita Y. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open 2015;5:e008058.



17. Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 2017;50:1602503PMID: 28954780.


18. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:87-94. PMID: 23460008.


19. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015;36:13-34. PMID: 25676516.


20. Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci 2018;33:e65. PMID: 29441757.



21. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 2006;129:341-348. PMID: 16478850.


22. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010;14:1069-1071. PMID: 20626955.

23. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis 2012;44:733-738. PMID: 22720876.


24. Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis 2012;72:409-415.

25. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest 2012;142:1482-1488. PMID: 22628488.


26. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017;49:1600537PMID: 28275170.


27. Pan SW, Shu CC, Feng JY, Wang JY, Chan YJ, Yu CJ, et al. Microbiological persistence in patients with Mycobacterium avium complex lung disease: the predictors and the Impact on radiographic progression. Clin Infect Dis 2017;65:927-934. PMID: 28541556.


28. Henkle E, Novosad SA, Shafer S, Hedberg K, Siegel SAR, Ku J, et al. Long-term outcomes in a population-based cohort with respiratory nontuberculous mycobacteria isolation. Ann Am Thorac Soc 2017;14:1120-1128. PMID: 28406709.



29. Kim SJ, Park J, Lee H, Lee YJ, Park JS, Cho YJ, et al. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis 2014;18:730-736. PMID: 24903946.


30. Kim SJ, Yoon SH, Choi SM, Lee J, Lee CH, Han SK, et al. Characteristics associated with progression in patients with of nontuberculous mycobacterial lung disease : a prospective cohort study. BMC Pulm Med 2017;17:5PMID: 28056937.



31. Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/Azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014;146:276-282. PMID: 24457542.



32. Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2015;191:96-103. PMID: 25393520.


33. Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc 2014;11:9-16. PMID: 24236749.



34. van Ingen J, Wagner D, Gallagher J, Morimoto K, Lange C, Haworth CS, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017;49:1601855PMID: 28182571.


35. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 2004;126:566-581. PMID: 15302746.


36. Xu HB, Jiang RH, Li L. Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2014;33:347-358. PMID: 23979729.


37. Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis 2017;65:1077-1084. PMID: 28582488.


38. Pasipanodya JG, Ogbonna D, Deshpande D, Srivastava S, Gumbo T. Meta-analyses and the evidence base for microbialoutcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother 2017;72(Suppl_2):i3-i19. PMID: 28922813.


39. Diel R, Nienhaus A, Ringshausen FC, Richter E, Welte T, Rabe KF, et al. Microbiologic outcome of interventions against Mycobacterium avium complex pulmonary disease: a systematic review. Chest 2018;153:888-921. PMID: 29410162.


40. Griffith DE, Adjemian J, Brown-Elliott BA, Philley JV, Prevots DR, Gaston C, et al. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2015;192:754-760. PMID: 26068042.



41. Loebinger MR. Mycobacterium avium complex infection: phenotypes and outcomes. Eur Respir J 2017;50:1701380PMID: 28954776.


42. Griffith DE, Brown-Elliott BA, Shepherd S, McLarty J, Griffith L, Wallace RJ Jr. Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2005;172:250-253. PMID: 15860751.


43. Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006;173:1283-1289. PMID: 16514112.


44. Jeong BH, Jeon K, Park HY, Moon SM, Kim SY, Lee SY, et al. Peak plasma concentration of azithromycin and treatment responses in Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 2016;60:6076-6083. PMID: 27480854.



45. Philley JV, Griffith DE. Treatment of slowly growing mycobacteria. Clin Chest Med 2015;36:79-90. PMID: 25676521.


46. Jhun BW, Kim SY, Moon SM, Jeon K, Kwon OJ, Huh HJ, et al. Development of macrolide resistance and reinfection in refractory Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2018;198:1322-1330. PMID: 29877739.


47. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT, Onyi GO, et al. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med 1994;149:1335-1341. PMID: 8173775.


48. Griffith DE, Brown BA, Girard WM, Murphy DT, Wallace RJ Jr. Azithromycin activity against Mycobacterium avium complex lung disease in patients who were not infected with human immunodeficiency virus. Clin Infect Dis 1996;23:983-989. PMID: 8922790.


49. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med 1996;153(6 Pt 1):1766-1772. PMID: 8665032.


50. Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ Jr. Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J Infect Dis 1998;178:121-126. PMID: 9652431.


51. Jhun BW, Moon SM, Kim SY, Park HY, Jeon K, Kwon OJ, et al. Intermittent antibiotic therapy for recurrent nodular bronchiectatic Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 2018;e01812-17. PMID: 29203483.



52. Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006;174:928-934. PMID: 16858014.


53. Kadota T, Matsui H, Hirose T, Suzuki J, Saito M, Akaba T, et al. Analysis of drug treatment outcome in clarithromycinresistant Mycobacterium avium complex lung disease. BMC Infect Dis 2016;16:31PMID: 26818764.


54. Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 2016;60:6758-6765. PMID: 27572413.



55. Morimoto K, Namkoong H, Hasegawa N, Nakagawa T, Morino E, Shiraishi Y, et al. Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc 2016;13:1904-1911. PMID: 27513168.


56. Koh WJ, Hong G, Kim SY, Jeong BH, Park HY, Jeon K, et al. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother 2013;57:2281-2285. PMID: 23478956.



57. Kang HK, Park HY, Kim D, Jeong BH, Jeon K, Cho JH, et al. Treatment outcomes of adjuvant resectional surgery for nontuberculous mycobacterial lung disease. BMC Infect Dis 2015;15:76PMID: 25887191.



58. Asakura T, Hayakawa N, Hasegawa N, Namkoong H, Takeuchi K, Suzuki S, et al. Long-term outcome of pulmonary resection for nontuberculous mycobacterial pulmonary disease. Clin Infect Dis 2017;65:244-251. PMID: 28369361.


59. Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-1892. PMID: 18498789.


60. Mitchell JD. Surgical approach to pulmonary nontuberculous mycobacterial infections. Clin Chest Med 2015;36:117-122. PMID: 25676524.


61. Tang S, Yao L, Hao X, Liu Y, Zeng L, Liu G, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis 2015;60:1361-1367. PMID: 25605283.


62. Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 2003;124:1482-1486. PMID: 14555583.


63. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 2016;149:1285-1293. PMID: 26513209.


64. Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 2017;152:800-809. PMID: 28483608.


65. Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 2014;11:30-35. PMID: 24460437.



66. Yagi K, Ishii M, Namkoong H, Asami T, Iketani O, Asakura T, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis 2017;17:558PMID: 28793869.



67. Jhun BW, Yang B, Moon SM, Lee H, Park HY, Jeon K, et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob Agents Chemother 2018;e00011-18. PMID: 29661870.



68. Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One 2014;9:e108703. PMID: 25264757.



69. Olivier KN, Griffith DE, Eagle G, McGinnis JP 2nd, Micioni L, Liu K, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017;195:814-823. PMID: 27748623.



70. Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT): a prospective, open-label, randomized study. Am J Respir Crit Care Med 2018 9 14 [Epub]. PMID: 10.1164/rccm.201807-1318OC.
71. Kwon YS, Jeong BH, Koh WJ. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med 2014;20:280-286. PMID: 24614239.


72. Kwon YS, Koh WJ. Synthetic investigational new drugs for the treatment of tuberculosis. Expert Opin Investig Drugs 2016;25:183-193.


73. Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ Jr. In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 2017;61:e01798-16. PMID: 27872065.



74. Vesenbeckh S, Schonfeld N, Krieger D, Bettermann G, Bauer TT, Russmann H, et al. Bedaquiline as a potential agent in the treatment of M. intracellulare and M. avium infections. Eur Respir J 2017;49:1601969PMID: 28331039.


75. Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015;148:499-506. PMID: 25675393.



76. van Ingen J, Aksamit T, Andrejak C, Bottger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 2018;51:1800170PMID: 29567726.



77. Satta G, McHugh TD, Mountford J, Abubakar I, Lipman M. Managing pulmonary nontuberculous mycobacterial infection. time for a patient-centered approach. Ann Am Thorac Soc 2014;11:117-121. PMID: 24460445.



78. Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med 2011;105:1718-1725. PMID: 21868209.


79. Lee MR, Yang CY, Chang KP, Keng LT, Yen DH, Wang JY, et al. Factors associated with lung function decline in patients with non-tuberculous mycobacterial pulmonary disease. PLoS One 2013;8:e58214. PMID: 23483998.



80. Park HY, Jeong BH, Chon HR, Jeon K, Daley CL, Koh WJ. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest 2016;150:1222-1232. PMID: 27298072.


81. Asakura T, Funatsu Y, Ishii M, Namkoong H, Yagi K, Suzuki S, et al. Health-related quality of life is inversely correlated with C-reactive protein and age in Mycobacterium avium complex lung disease: a cross-sectional analysis of 235 patients. Respir Res 2015;16:145PMID: 26635226.



82. Hama M, Ushiki A, Kosaka M, Yamazaki Y, Yasuo M, Yamamoto H, et al. Health-related quality of life in patients with pulmonary non-tuberculous mycobacteria infection. Int J Tuberc Lung Dis 2016;20:747-752. PMID: 27155176.


83. Asakura T, Ishii M, Ishii K, Suzuki S, Namkoong H, Okamori S, et al. Health-related QOL of elderly patients with pulmonary M. avium complex disease in a university hospital. Int J Tuberc Lung Dis 2018;22:695-703. PMID: 29862956.


84. Czaja CA, Levin AR, Cox CW, Vargas D, Daley CL, Cott GR. Improvement in quality of life after therapy for Mycobacterium abscessus group lung infection: a prospective cohort study. Ann Am Thorac Soc 2016;13:40-48. PMID: 26540302.


Figure┬Ā1
Treatment initiation algorithm for treatment naïve MAC-PD. Treatment should be considered when patients have risk factors for disease progression, including cavitary lesion(s), low body mass index, poor nutritional status, extensive disease, and AFB smear-positive sputum. If patients have mild disease and no risk factors for progression, treatment should be initiated when patients exhibit disease progression. MAC-PD: Mycobacterium avium complex pulmonary disease; HRCT: high-resolution computed tomography; AFB: acid-fast bacilli.
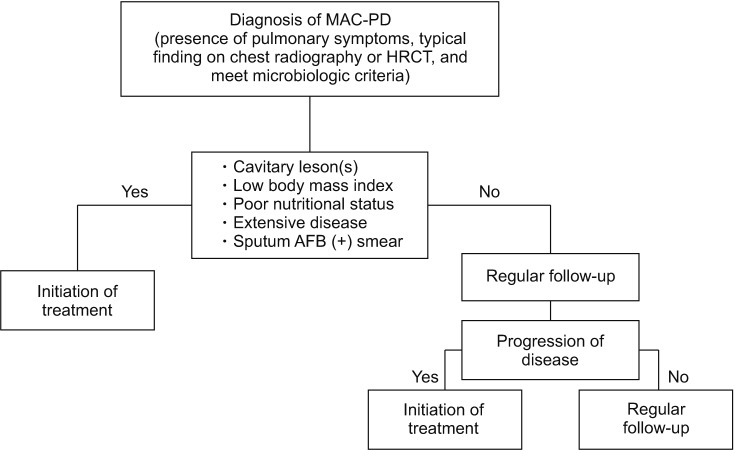
Figure┬Ā2
Fibrocavitary form of Mycobacterium intracellulare pulmonary disease in a 57-year-old male patient. (A) Chest high-resolution computed tomography (HRCT) shows a large, thick-walled cavity in the right upper lobe. (B) After 12 months of daily azithromycin, ethambutol, and rifampin treatment in combination with streptomycin injection for the initial 3 months, chest HRCT showed improvement of the cavitary lesion.
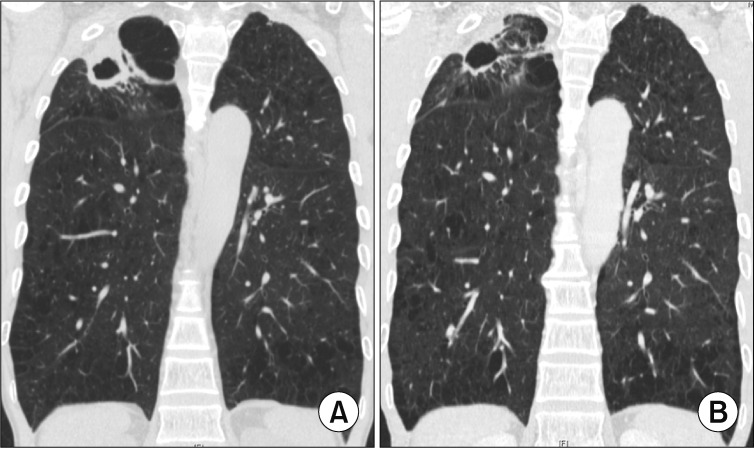
Figure┬Ā3
Cavitary nodular bronchiectatic form of Mycobacterium avium pulmonary disease in a 61-year-old female patient. (A) Chest high-resolution computed tomography (HRCT) shows severe bronchiectasis in the lingular segment of the left upper lobe. Note the cavity and multiple nodules suggesting bronchiolitis in the left lower lobe. (B) After 12 months of daily azithromycin, ethambutol, and rifampin treatment, chest HRCT showed improvement of cavitary and nodular lesions.

Figure┬Ā4
Non-cavitary nodular bronchiectatic form of pulmonary disease caused by Mycobacterium intracellulare in a 57-year-old female patient. (A) Chest high-resolution computed tomography (HRCT) shows severe bronchiectasis in the right middle lobe and the lingular segment of the left upper lobe. Note the peribronchiectatic consolidation, the multiple, small nodules, and tree-in-bud appearances suggesting bronchiolitis in both lungs. (B) After 12 months of three-times-weekly antibiotic therapy that included azithromycin, ethambutol, and rifampin, chest HRCT showed a decreased extent of bilateral consolidation and improvement of bronchiolitis.
Table┬Ā1
Summary of studies with meta-analysis on treatment outcomes of Mycobacterium avium complex pulmonary disease
| Study | No. of studies | No. of patients | Selection criteria of studies | Definition of treatment success | Treatment success rate (% or 95% CI) | Other outcomes |
|---|---|---|---|---|---|---|
| Field et al. (2004)35 | 38 | 1,152 | All | Variable | Prior to introduction of ethambutol or rifampin: 32 | |
| Regimens included ethambutol and/or rifampin: 39 | ||||||
| Macrolide-containing regimens: 56 | ||||||
| Xu et al. (2014)36 | 28 | 2,422 | Studies with cases of culture-confirmed MAC by ATS guidelines, outcome definitions specified by mycobacterial culture, and outcomes reported according to the ATS classifications | Variable, specified by mycobacterial culture endpoints | 39 (38-41) | Failure 27 (25.29) |
| Treatment success rate of regimens with macrolide vs. without macrolide, 42 (40-44) vs. 28 (24-32) | Relapse 6 (5.7) | |||||
| Death 17 (15.18) | ||||||
| Default 12 (11.13) | ||||||
| Kwak et al. (2017)37 | 16 | 1,462 | Randomized, controlled trials and observational studies published in peer-reviewed journals | 12 Months of sustained culture negativity | 60 (55-65) | Default rate of all studies vs. studies with tiw dosing: 16 (12-20) vs. 12 (9-15) |
| Pasipanodya et al. (2017)38 | 21 | 2,534 | Clinical trials, prospective and retrospective studies | Sputum culture conversion | 6-Month culture conversion with macrolide-containing regimen: 65 (58-72) | |
| Culture conversion at the end of treatment with macrolide-containing regimens: 64 (55-73) | ||||||
| Culture conversion at the end of treatment with macrolide-free regimens: 53 (15-89) | ||||||
| Sustained culture conversion with macrolide-containing regimens: 54 (45-63) | ||||||
| Sustained culture conversion with macrolide-free regimens: 38 (25-52) | ||||||
| Diel et al. (2018)39 | 42 | 2,376 | Clinical trials, prospective and retrospective studies | Sputum culture conversion | Culture conversion with macrolidecontaining regimen: 52 (45-60) | |
| Culture conversion with ATSrecommended three-drug regimen: 61 (50-73) | ||||||
| Culture conversion with ATSrecommended three-drug regimen after more than 1 year: 66 (53-77) |
Table┬Ā2
Treatment of Mycobacterium avium complex pulmonary disease
Table┬Ā3
International expert consensus on treatment outcome definitions for non-tuberculous mycobacterial pulmonary diseases
Reprinted from van Ingen et al. Eur Respir J 2018;51:1800170, with permission of the European Respiratory Society76.
NTM-PD: nontuberculous mycobacterial pulmonary disease.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation